Ongoing Notch signaling maintains phenotypic fidelity in the adult exocrine pancreas
- PMID: 22146645
- PMCID: PMC3254730
- DOI: 10.1016/j.ydbio.2011.11.010
Ongoing Notch signaling maintains phenotypic fidelity in the adult exocrine pancreas
Abstract
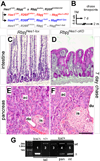
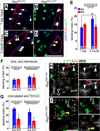
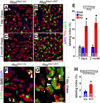
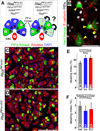
The Notch signaling pathway regulates embryonic development of the pancreas, inhibiting progenitor differentiation into exocrine acinar and endocrine islet cells. The adult pancreas appears to lack progenitor cells, and its mature cell types are maintained by the proliferation of pre-existing differentiated cells. Nonetheless, Notch remains active in adult duct and terminal duct/centroacinar cells (CACs), in which its function is unknown. We previously developed mice in which cells expressing the Notch target gene Hes1 can be labeled and manipulated, by expression of Cre recombinase, and demonstrated that Hes1(+) CACs do not behave as acinar or islet progenitors in the uninjured pancreas, or as islet progenitors after pancreatic duct ligation. In the current study, we assessed the function of Notch signaling in the adult pancreas by deleting the transcription factor partner of Notch, Rbpj, specifically in Hes1(+) cells. We find that loss of Rbpj depletes the pancreas of Hes1-expressing CACs, abrogating their ongoing contribution to growth and homeostasis of more proximal duct structures. Upon Rbpj deletion, CACs undergo a rapid transformation into acinar cells, suggesting that constitutive Notch activity suppresses the acinar differentiation potential of CACs. Together, our data provide direct evidence of an endogenous genetic program to control interconversion of cell fates in the adult pancreas.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Impact of Sox9 dosage and Hes1-mediated Notch signaling in controlling the plasticity of adult pancreatic duct cells in mice.Sci Rep. 2015 Feb 17;5:8518. doi: 10.1038/srep08518. Sci Rep. 2015. PMID: 25687338 Free PMC article.
-
Lineage tracing reveals the dynamic contribution of Hes1+ cells to the developing and adult pancreas.Development. 2011 Feb;138(3):431-41. doi: 10.1242/dev.053843. Development. 2011. PMID: 21205788 Free PMC article.
-
Ectopic pancreas formation in Hes1 -knockout mice reveals plasticity of endodermal progenitors of the gut, bile duct, and pancreas.J Clin Invest. 2006 Jun;116(6):1484-93. doi: 10.1172/JCI27704. Epub 2006 May 18. J Clin Invest. 2006. PMID: 16710472 Free PMC article.
-
Rhythmic gene expression in somite formation and neural development.Mol Cells. 2009 May 31;27(5):497-502. doi: 10.1007/s10059-009-0068-1. Epub 2009 May 15. Mol Cells. 2009. PMID: 19466597 Review.
-
Molecular and cellular regulation of pancreatic acinar cell function.Curr Opin Gastroenterol. 2009 Sep;25(5):466-71. doi: 10.1097/MOG.0b013e32832ebfac. Curr Opin Gastroenterol. 2009. PMID: 19571746 Free PMC article. Review.
Cited by
-
Dendritic Inhibition by Shh Signaling-Dependent Stellate Cell Pool Is Critical for Motor Learning.J Neurosci. 2022 Jun 29;42(26):5130-5143. doi: 10.1523/JNEUROSCI.2073-21.2022. Epub 2022 May 19. J Neurosci. 2022. PMID: 35589396 Free PMC article.
-
Ciliary Hedgehog Signaling Restricts Injury-Induced Adipogenesis.Cell. 2017 Jul 13;170(2):340-351.e12. doi: 10.1016/j.cell.2017.06.035. Cell. 2017. PMID: 28709001 Free PMC article.
-
Loss of Pten and Activation of Kras Synergistically Induce Formation of Intraductal Papillary Mucinous Neoplasia From Pancreatic Ductal Cells in Mice.Gastroenterology. 2018 Apr;154(5):1509-1523.e5. doi: 10.1053/j.gastro.2017.12.007. Epub 2017 Dec 19. Gastroenterology. 2018. PMID: 29273451 Free PMC article.
-
Control of cell identity in pancreas development and regeneration.Gastroenterology. 2013 Jun;144(6):1170-9. doi: 10.1053/j.gastro.2013.01.074. Gastroenterology. 2013. PMID: 23622126 Free PMC article. Review.
-
Impact of Sox9 dosage and Hes1-mediated Notch signaling in controlling the plasticity of adult pancreatic duct cells in mice.Sci Rep. 2015 Feb 17;5:8518. doi: 10.1038/srep08518. Sci Rep. 2015. PMID: 25687338 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

