3-dimensional imaging modalities for phenotyping genetically engineered mice
- PMID: 22146851
- PMCID: PMC3857693
- DOI: 10.1177/0300985811429814
3-dimensional imaging modalities for phenotyping genetically engineered mice
Abstract
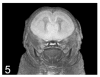
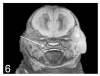
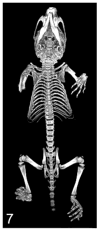
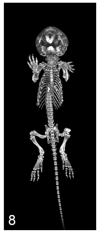
A variety of 3-dimensional (3D) digital imaging modalities are available for whole-body assessment of genetically engineered mice: magnetic resonance microscopy (MRM), X-ray microcomputed tomography (microCT), optical projection tomography (OPT), episcopic and cryoimaging, and ultrasound biomicroscopy (UBM). Embryo and adult mouse phenotyping can be accomplished at microscopy or near microscopy spatial resolutions using these modalities. MRM and microCT are particularly well-suited for evaluating structural information at the organ level, whereas episcopic and OPT imaging provide structural and functional information from molecular fluorescence imaging at the cellular level. UBM can be used to monitor embryonic development longitudinally in utero. Specimens are not significantly altered during preparation, and structures can be viewed in their native orientations. Technologies for rapid automated data acquisition and high-throughput phenotyping have been developed and continually improve as this exciting field evolves.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Wilson has a financial interest in BioInVision, Inc.
Figures
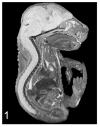
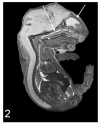
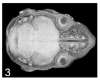
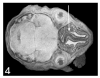
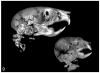
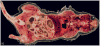

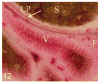

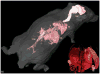
Similar articles
-
Imaging modalities to assess structural birth defects in mutant mouse models.Birth Defects Res C Embryo Today. 2010 Sep;90(3):176-84. doi: 10.1002/bdrc.20187. Birth Defects Res C Embryo Today. 2010. PMID: 20860057 Review.
-
Embryonic and neonatal phenotyping of genetically engineered mice.ILAR J. 2006;47(2):103-17. doi: 10.1093/ilar.47.2.103. ILAR J. 2006. PMID: 16547367 Review.
-
Label-free optical projection tomography for quantitative three-dimensional anatomy of mouse embryo.J Biophotonics. 2019 Jul;12(7):e201800481. doi: 10.1002/jbio.201800481. Epub 2019 Apr 29. J Biophotonics. 2019. PMID: 30729697
-
A feasibility study of OCT for anatomical and vascular phenotyping of mouse embryo.J Biophotonics. 2020 May;13(5):e201960225. doi: 10.1002/jbio.201960225. Epub 2020 Mar 1. J Biophotonics. 2020. PMID: 32067352
-
microMRI-HREM pipeline for high-throughput, high-resolution phenotyping of murine embryos.J Anat. 2007 Jul;211(1):132-7. doi: 10.1111/j.1469-7580.2007.00746.x. Epub 2007 May 28. J Anat. 2007. PMID: 17532797 Free PMC article.
Cited by
-
Automatic Stem Cell Detection in Microscopic Whole Mouse Cryo-Imaging.IEEE Trans Med Imaging. 2016 Mar;35(3):819-29. doi: 10.1109/TMI.2015.2497285. Epub 2015 Nov 2. IEEE Trans Med Imaging. 2016. PMID: 26552080 Free PMC article.
-
Optimization of Pulmonary Vasculature Tridimensional Phenotyping in The Rat Fetus.Sci Rep. 2019 Feb 4;9(1):1244. doi: 10.1038/s41598-018-37906-8. Sci Rep. 2019. PMID: 30718645 Free PMC article.
-
High-resolution Episcopic Microscopy (HREM) - Simple and Robust Protocols for Processing and Visualizing Organic Materials.J Vis Exp. 2017 Jul 7;(125):56071. doi: 10.3791/56071. J Vis Exp. 2017. PMID: 28715372 Free PMC article.
-
Morphometrics, 3D Imaging, and Craniofacial Development.Curr Top Dev Biol. 2015;115:561-97. doi: 10.1016/bs.ctdb.2015.09.003. Epub 2015 Oct 27. Curr Top Dev Biol. 2015. PMID: 26589938 Free PMC article. Review.
-
Resolving complex cartilage structures in developmental biology via deep learning-based automatic segmentation of X-ray computed microtomography images.Sci Rep. 2022 May 24;12(1):8728. doi: 10.1038/s41598-022-12329-8. Sci Rep. 2022. PMID: 35610276 Free PMC article.
References
-
- Ali AA, Dale AM, Badea A, et al. Automated segmentation of neuroanatomical structures in multispectral MR microscopy of the mouse brain. Neuroimage. 2005;27:425–435. - PubMed
-
- Antal C, Teletin M, Wendling O, et al. Tissue collection for systematic phenotyping in the mouse. Curr Protoc Mol Biol. 2007;(Unit 29A.4) - PubMed
-
- Aoyagi H, Tsuchikawa K, Iwasaki S. Three-dimensional observation of the mouse embryo by micro-computed tomography: composition of the trigeminal ganglion. Odontology. 2010;98:26–30. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

