Do urology journals enforce trial registration? A cross-sectional study of published trials
- PMID: 22146890
- PMCID: PMC3236819
- DOI: 10.1136/bmjopen-2011-000430
Do urology journals enforce trial registration? A cross-sectional study of published trials
Erratum in
-
Correction: Do urology journals enforce trial registration? A cross-sectional study of published trials.BMJ Open. 2018 Jan 24;8(1):e000430corr1. doi: 10.1136/bmjopen-2011-000430corr1. eCollection 2018. BMJ Open. 2018. PMID: 31329763 Free PMC article.
Abstract
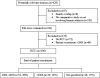
Objectives (1) To assess endorsement of trial registration in author instructions of urology-related journals and (2) to assess whether randomised controlled trials (RCTs) in the field of urology were effectively registered. Design Cross-sectional study of author instructions and published trials. Setting Journals publishing in the field of urology. Participants First, the authors analysed author instructions of 55 urology-related journals indexed in 'Journal Citation Reports 2009' (12/2010). The authors divided these journals in two groups: those requiring and those not mentioning trial registration as a precondition for publication. Second, the authors chose the five journals with the highest impact factor (IF) from each group. Intervention MEDLINE search to identify RCTs published in these 10 journals in 2009 (01/2011); search of the clinical trials meta-search interface of WHO (International Clinical Trials Registry Platform) for RCTs that lacked information about registration (01-03/2011). Two authors independently assessed the information. Outcome measures Proportion of journals providing advice about trial registration and proportion of trials registered. Results Of 55 journals analysed, 26 (47.3%) provided some editorial advice about trial registration. Journals with higher IFs were more likely to mention trial registration explicitly (p=0.015). Of 106 RCTs published in 2009, 63 were registered (59.4%) with a tendency to an increase after 2005 (83.3%, p=0.035). 71.4% (30/42) of the RCTs that were published in journals mentioning and requiring registration, and 51.6% (33/64) of the RCTs that were published in journals that did not mention trial registration explicitly were registered. This difference was statistically significant (p=0.04). Conclusions The existence of a statement about trial registration in author instructions resulted in a higher proportion of registered RCTs in those journals. Journals with higher IFs were more likely to mention trial registration.
Conflict of interest statement
Figures
Similar articles
-
Surgical trials and trial registers: a cross-sectional study of randomized controlled trials published in journals requiring trial registration in the author instructions.Trials. 2013 Dec 1;14:407. doi: 10.1186/1745-6215-14-407. Trials. 2013. PMID: 24289719 Free PMC article.
-
Endorsement of the CONSORT statement by high-impact medical journals in China: a survey of instructions for authors and published papers.PLoS One. 2012;7(2):e30683. doi: 10.1371/journal.pone.0030683. Epub 2012 Feb 13. PLoS One. 2012. PMID: 22348017 Free PMC article.
-
Prospective registration and reporting of trial number in randomised clinical trials: global cross sectional study of the adoption of ICMJE and Declaration of Helsinki recommendations.BMJ. 2020 Apr 14;369:m982. doi: 10.1136/bmj.m982. BMJ. 2020. PMID: 32291261 Free PMC article.
-
Do emergency medicine journals promote trial registration and adherence to reporting guidelines? A survey of "Instructions for Authors".Scand J Trauma Resusc Emerg Med. 2016 Nov 24;24(1):137. doi: 10.1186/s13049-016-0331-3. Scand J Trauma Resusc Emerg Med. 2016. PMID: 27881175 Free PMC article. Review.
-
The use of systematic reviews to justify anaesthesiology trials: A meta-epidemiological study.Eur J Pain. 2018 Nov;22(10):1844-1849. doi: 10.1002/ejp.1280. Epub 2018 Jul 24. Eur J Pain. 2018. PMID: 29978522
Cited by
-
Linking ClinicalTrials.gov and PubMed to track results of interventional human clinical trials.PLoS One. 2013 Jul 9;8(7):e68409. doi: 10.1371/journal.pone.0068409. Print 2013. PLoS One. 2013. PMID: 23874614 Free PMC article.
-
[Trial registration for the improvement of transparency in scientific research. The German Association of Urology now enables registration in the register network of the WHO].Urologe A. 2012 Sep;51(9):1278-81. doi: 10.1007/s00120-012-2914-6. Urologe A. 2012. PMID: 22695977 German.
-
Evaluating guideline and registration policies among neurology journals: a cross-sectional analysis.BMC Neurol. 2024 Sep 5;24(1):321. doi: 10.1186/s12883-024-03839-1. BMC Neurol. 2024. PMID: 39237894 Free PMC article.
-
Is Mandatory Prospective Trial Registration Working to Prevent Publication of Unregistered Trials and Selective Outcome Reporting? An Observational Study of Five Psychiatry Journals That Mandate Prospective Clinical Trial Registration.PLoS One. 2015 Aug 19;10(8):e0133718. doi: 10.1371/journal.pone.0133718. eCollection 2015. PLoS One. 2015. PMID: 26287998 Free PMC article.
-
A cross-sectional study on the endorsement of reporting guidelines and clinical trial registration among immunology and allergy journals.PLoS One. 2025 May 7;20(5):e0322003. doi: 10.1371/journal.pone.0322003. eCollection 2025. PLoS One. 2025. PMID: 40333833 Free PMC article.
References
-
- Dickersin K, Chan S, Chalmers TC, et al. Publication bias and clinical trials. Control Clin Trials 1987;8:343–53 - PubMed
-
- Wildt S, Krag A, Gluud L. Characteristics of randomised trials on diseases in the digestive system registered in ClinicalTrials.gov: a retrospective analysis. BMJ Open 2011;1:e000309 - PMC - PubMed
-
- De Angelis C, Drazen JM, Frizelle FA, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. Ann Intern Med 2004;141:477–8 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous