An immune-active tumor microenvironment favors clinical response to ipilimumab
- PMID: 22146893
- PMCID: PMC11028506
- DOI: 10.1007/s00262-011-1172-6
An immune-active tumor microenvironment favors clinical response to ipilimumab
Abstract
Purpose: Ipilimumab, a fully human monoclonal antibody specific to CTLA-4, has been shown to improve overall survival in metastatic melanoma patients. As a consequence of CTLA-4 blockade, ipilimumab treatment is associated with proliferation and activation of peripheral T cells. To better understand various tumor-associated components that may influence the clinical outcome of ipilimumab treatment, gene expression profiles of tumors from patients treated with ipilimumab were characterized.
Experimental design: Gene expression profiling was performed on tumor biopsies collected from 45 melanoma patients before and 3 weeks after the start of treatment in a phase II clinical trial.
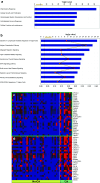
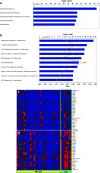
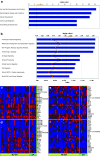
Results: Analysis of pre-treatment tumors indicated that patients with high baseline expression levels of immune-related genes were more likely to respond favorably to ipilimumab. Furthermore, ipilimumab appeared to induce two major changes in tumors from patients who exhibited clinical activity: genes involved in immune response showed increased expression, whereas expression of genes for melanoma-specific antigens and genes involved in cell proliferation decreased. These changes were associated with the total lymphocyte infiltrate in tumors, and there was a suggestion of association with prolonged overall survival in these patients. Many IFN-γ-inducible genes and Th1-associated markers showed increased expression after ipilimumab treatment, suggesting an accumulation of this particular type of T cell at the tumor sites, which might play an important role in mediating the antitumor activity of ipilimumab.
Conclusions: These results support the proposed mechanism of action of ipilimumab, suggesting that cell-mediated immune responses play an important role in the antitumor activity of ipilimumab.
Conflict of interest statement
Rui-Ru Ji, Scott D. Chasalow, Lisu Wang, John Cogswell, Suresh Alaparthy, David Berman, Maria Jure-Kunkel, Nathan O. Siemers, Jeffrey R. Jackson, and Vafa Shahabi are employees of Bristol-Myers Squibb, the manufacturer of ipilimumab.
Figures

References
-
- American Cancer Society (2009) Cancer facts and figures 2009. http://www.cancer.org/acs/groups/content/@nho/documents/document/500809w...
-
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK (eds). SEER Cancer Statistics Review, 1975–2008, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011
-
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985, 1993. J Clin Oncol. 1999;17(7):2105–2116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

