When to wait for more evidence? Real options analysis in proton therapy
- PMID: 22147003
- PMCID: PMC3248774
- DOI: 10.1634/theoncologist.2011-0029
When to wait for more evidence? Real options analysis in proton therapy
Abstract
Purpose: Trends suggest that cancer spending growth will accelerate. One method for controlling costs is to examine whether the benefits of new technologies are worth the extra costs. However, especially new and emerging technologies are often more costly, while limited clinical evidence of superiority is available. In that situation it is often unclear whether to adopt the new technology now, with the risk of investing in a suboptimal therapy, or to wait for more evidence, with the risk of withholding patients their optimal treatment. This trade-off is especially difficult when it is costly to reverse the decision to adopt a technology, as is the case for proton therapy. Real options analysis, a technique originating from financial economics, assists in making this trade-off.
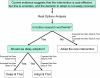
Methods: We examined whether to adopt proton therapy, as compared to stereotactic body radiotherapy, in the treatment of inoperable stage I non-small cell lung cancer. Three options are available: adopt without further research; adopt and undertake a trial; or delay adoption and undertake a trial. The decision depends on the expected net gain of each option, calculated by subtracting its total costs from its expected benefits.
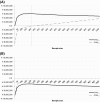
Results: In The Netherlands, adopt and trial was found to be the preferred option, with an optimal sample size of 200 patients. Increase of treatment costs abroad and costs of reversal altered the preferred option.
Conclusion: We have shown that real options analysis provides a transparent method of weighing the costs and benefits of adopting and/or further researching new and expensive technologies.
Conflict of interest statement
Figures


Comment in
-
Making investments in medical technology: time to get real about real options.Oncologist. 2011;16(12):1672-4. doi: 10.1634/theoncologist.2011-0345. Epub 2011 Dec 6. Oncologist. 2011. PMID: 22147001 Free PMC article.
Similar articles
-
A comparison of surgical intervention and stereotactic body radiation therapy for stage I lung cancer in high-risk patients: a decision analysis.J Thorac Cardiovasc Surg. 2012 Feb;143(2):428-36. doi: 10.1016/j.jtcvs.2011.10.078. Epub 2011 Dec 9. J Thorac Cardiovasc Surg. 2012. PMID: 22169443
-
Cost-effectiveness of stereotactic body radiation therapy versus video assisted thoracic surgery in medically operable stage I non-small cell lung cancer: A modeling study.Lung Cancer. 2020 Mar;141:89-96. doi: 10.1016/j.lungcan.2020.01.011. Epub 2020 Jan 16. Lung Cancer. 2020. PMID: 31982640
-
The cost-effectiveness of particle therapy in non-small cell lung cancer: exploring decision uncertainty and areas for future research.Cancer Treat Rev. 2010 Oct;36(6):468-76. doi: 10.1016/j.ctrv.2010.02.018. Epub 2010 Mar 29. Cancer Treat Rev. 2010. PMID: 20303217
-
Role of stereotactic body radiation therapy and proton/carbon nuclei therapies.Cancer J. 2013 May-Jun;19(3):272-81. doi: 10.1097/PPO.0b013e31829452a5. Cancer J. 2013. PMID: 23708074 Review.
-
Video assisted thoracoscopic surgery and lobectomy, sublobar resection, radiofrequency ablation, and stereotactic radiosurgery: advances and controversies in the management of early stage non-small cell lung cancer.Curr Opin Pulm Med. 2007 Jul;13(4):267-70. doi: 10.1097/MCP.0b013e3281c61a85. Curr Opin Pulm Med. 2007. PMID: 17534171 Review.
Cited by
-
Coverage with evidence development schemes for medical devices in Europe: characteristics and challenges.Eur J Health Econ. 2021 Nov;22(8):1253-1273. doi: 10.1007/s10198-021-01334-9. Epub 2021 Jun 12. Eur J Health Econ. 2021. PMID: 34117987 Free PMC article.
-
Sample Size Estimation for Non-Inferiority Trials: Frequentist Approach versus Decision Theory Approach.PLoS One. 2015 Jun 15;10(6):e0130531. doi: 10.1371/journal.pone.0130531. eCollection 2015. PLoS One. 2015. PMID: 26076354 Free PMC article.
-
Decision analytic modeling for the economic analysis of proton radiotherapy for non-small cell lung cancer.Transl Lung Cancer Res. 2018 Apr;7(2):122-133. doi: 10.21037/tlcr.2018.03.27. Transl Lung Cancer Res. 2018. PMID: 29876311 Free PMC article.
-
Healthy decisions: towards uncertainty tolerance in healthcare policy.Pharmacoeconomics. 2015 Jan;33(1):1-4. doi: 10.1007/s40273-014-0201-7. Pharmacoeconomics. 2015. PMID: 25145801 No abstract available.
-
Making investments in medical technology: time to get real about real options.Oncologist. 2011;16(12):1672-4. doi: 10.1634/theoncologist.2011-0345. Epub 2011 Dec 6. Oncologist. 2011. PMID: 22147001 Free PMC article.
References
-
- Meropol NJ, Schrag D, Smith TJ, et al. American Society of Clinical Oncology guidance statement: the cost of cancer care. J Clin Oncol. 2009;27:3868–3874. - PubMed
-
- Truffer CJ, Keehan S, Smith S, et al. Health spending projections through 2019: the recession's impact continues. Health Aff (Millwood) 2010;29:522–529. - PubMed
-
- Zietman A, Goitein M, Tepper JE. Technology evolution: is it survival of the fittest? J Clin Oncol. 2010;28:4275–4279. - PubMed
-
- Drummond MF, Sculpher MJ, Torrance GW, et al. 3rd ed. New York: Oxford University Press; 2005. Methods for the Economic Evaluation of Health Care Programmes.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

