Liganded thyroid hormone receptor induces nucleosome removal and histone modifications to activate transcription during larval intestinal cell death and adult stem cell development
- PMID: 22147009
- PMCID: PMC3275393
- DOI: 10.1210/en.2011-1736
Liganded thyroid hormone receptor induces nucleosome removal and histone modifications to activate transcription during larval intestinal cell death and adult stem cell development
Abstract
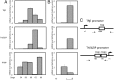
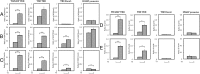
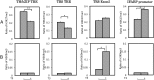
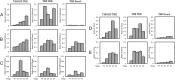
Thyroid hormone (T(3)) plays an important role in regulating multiple cellular and metabolic processes, including cell proliferation, cell death, and energy metabolism, in vertebrates. Dysregulation of T(3) signaling results in developmental abnormalities, metabolic defects, and even cancer. We used T(3)-dependent Xenopus metamorphosis as a model to study how T(3) regulates transcription during vertebrate development. T(3) exerts its metamorphic effects through T(3) receptors (TR). TR recruits, in a T(3)-dependent manner, cofactor complexes that can carry out chromatin remodeling/histone modifications. Whether and how histone modifications change upon gene regulation by TR during vertebrate development is largely unknown. Here we analyzed histone modifications at T(3) target genes during intestinal metamorphosis, a process that involves essentially total apoptotic degeneration of the simple larval epithelium and de novo development of the adult epithelial stem cells, followed by their proliferation and differentiation into the complex adult epithelium. We demonstrated for the first time in vivo during vertebrate development that TR induces the removal of core histones at the promoter region and the recruitment of RNA polymerase. Furthermore, a number of histone activation and repression marks have been defined based on correlations with mRNA levels in cell cultures. Most but not all correlate with gene expression induced by liganded TR during development, suggesting that tissue and developmental context influences the roles of histone modifications in gene regulation. Our findings provide important mechanistic insights on how chromatin remodeling affects developmental gene regulation in vivo.
Figures



Similar articles
-
Involvement of epigenetic modifications in thyroid hormone-dependent formation of adult intestinal stem cells during amphibian metamorphosis.Gen Comp Endocrinol. 2019 Jan 15;271:91-96. doi: 10.1016/j.ygcen.2018.11.012. Epub 2018 Nov 22. Gen Comp Endocrinol. 2019. PMID: 30472386 Free PMC article.
-
Novel functions of protein arginine methyltransferase 1 in thyroid hormone receptor-mediated transcription and in the regulation of metamorphic rate in Xenopus laevis.Mol Cell Biol. 2009 Feb;29(3):745-57. doi: 10.1128/MCB.00827-08. Epub 2008 Dec 1. Mol Cell Biol. 2009. PMID: 19047371 Free PMC article.
-
A Role of Endogenous Histone Acetyltransferase Steroid Hormone Receptor Coactivator 3 in Thyroid Hormone Signaling During Xenopus Intestinal Metamorphosis.Thyroid. 2021 Apr;31(4):692-702. doi: 10.1089/thy.2020.0410. Epub 2020 Nov 20. Thyroid. 2021. PMID: 33076783 Free PMC article.
-
Corepressor requirement and thyroid hormone receptor function during Xenopus development.Vitam Horm. 2004;68:209-30. doi: 10.1016/S0083-6729(04)68007-1. Vitam Horm. 2004. PMID: 15193456 Review.
-
Mechanisms of thyroid hormone receptor action during development: lessons from amphibian studies.Biochim Biophys Acta. 2013 Jul;1830(7):3882-92. doi: 10.1016/j.bbagen.2012.04.020. Epub 2012 Apr 28. Biochim Biophys Acta. 2013. PMID: 22565053 Review.
Cited by
-
Organ-Specific Requirements for Thyroid Hormone Receptor Ensure Temporal Coordination of Tissue-Specific Transformations and Completion of Xenopus Metamorphosis.Thyroid. 2020 Feb;30(2):300-313. doi: 10.1089/thy.2019.0366. Epub 2020 Jan 23. Thyroid. 2020. PMID: 31854240 Free PMC article.
-
Insufficiency of Thyroid Hormone in Frog Metamorphosis and the Role of Glucocorticoids.Front Endocrinol (Lausanne). 2019 May 9;10:287. doi: 10.3389/fendo.2019.00287. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31143159 Free PMC article. Review.
-
Histone methyltransferase Dot1L is a coactivator for thyroid hormone receptor during Xenopus development.FASEB J. 2017 Nov;31(11):4821-4831. doi: 10.1096/fj.201700131R. Epub 2017 Jul 24. FASEB J. 2017. PMID: 28739643 Free PMC article.
-
Histone methyltransferase Dot1L plays a role in postembryonic development in Xenopus tropicalis.FASEB J. 2015 Feb;29(2):385-93. doi: 10.1096/fj.14-252171. Epub 2014 Nov 3. FASEB J. 2015. PMID: 25366346 Free PMC article.
-
Thyroid hormone receptor subtype-specific function in controlling organ-specific developmental timing and rate during Xenopus development.Front Endocrinol (Lausanne). 2025 Jun 9;16:1614439. doi: 10.3389/fendo.2025.1614439. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40551888 Free PMC article. Review.
References
-
- Lazar MA. 1993. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev 14:184–193 - PubMed
-
- Yen PM. 2001. Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 - PubMed
-
- Tata JR. 1993. Gene expression during metamorphosis: an ideal model for post-embryonic development. Bioessays 15:239–248 - PubMed
-
- Shi Y-B. 1999. Amphibian metamorphosis: from morphology to molecular biology. New York: John Wiley, Sons, Inc
-
- Hetzel BS. 1989. The story of iodine deficiency: an international challenge in nutrition. Oxford, UK: Oxford University Press

