Differential responses of progesterone receptor membrane component-1 (Pgrmc1) and the classical progesterone receptor (Pgr) to 17β-estradiol and progesterone in hippocampal subregions that support synaptic remodeling and neurogenesis
- PMID: 22147012
- PMCID: PMC3275384
- DOI: 10.1210/en.2011-1699
Differential responses of progesterone receptor membrane component-1 (Pgrmc1) and the classical progesterone receptor (Pgr) to 17β-estradiol and progesterone in hippocampal subregions that support synaptic remodeling and neurogenesis
Abstract
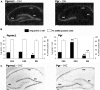
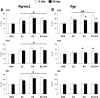
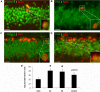
Progesterone (P4) and estradiol (E2) modulate neurogenesis and synaptic remodeling in the hippocampus during the rat estrous cycle and in response to deafferenting lesions, but little is known about the steroidal regulation of hippocampal progesterone receptors associated with these processes. We examined the neuronal expression of progesterone receptor membrane component-1 (Pgrmc1) and the classical progesterone receptor (Pgr), by in situ hybridization and immunohistochemistry. Pgr, a transcription factor, has been associated with synaptic remodeling and other major actions of P4, whereas Pgrmc1 is implicated in P4-dependent proliferation of adult neuroprogenitor cells and with rapid P4 effects on membranes. Ovariectomized adult rats were given E2, P4, or E2+P4 on two schedules: a 4-d model of the rodent estrous cycle and a 30-d model of postmenopausal hormone therapy. Pgr was hormonally responsive only in CA1 pyramidal neurons, and the induction of Pgr by E2 was partly antagonized by P4 only on the 30-d schedule. In CA3 pyramidal and dentate gyrus (DG) neurons, Pgr was largely unresponsive to all hormone treatments. In contrast to Pgr, Pgrmc1 was generally induced by E2 and/or P4 throughout the hippocampus in CA1, CA3, and DG neurons. In neuroprogenitor cells of the DG (immunopositive for bromodeoxyuridine and doublecortin), both Pgrmc1 and Pgr were detected. The differential regulation of hippocampal Pgrmc1 and Pgr by E2 and P4 may guide drug development in hormonal therapy for support of neurogenesis and synaptic regeneration.
Figures

References
-
- Graham JD, Clarke CL. 1997. Physiological action of progesterone in target tissues. Endocr Rev 18:502–519 - PubMed
-
- Rosario G, Sachdeva G, Okulicz WC, Ace CI, Katkam RR, Puri CP. 2003. Role of progesterone in structural and biochemical remodeling of endometrium. Front Biosci 8:s924–s935 - PubMed
-
- Woolley CS, McEwen BS. 1993. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol 336:293–306 - PubMed
-
- Cooke BM, Woolley CS. 2005. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol 64:34–46 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

