Phosphatase and tensin homologue (PTEN) regulates synaptic plasticity independently of its effect on neuronal morphology and migration
- PMID: 22147265
- PMCID: PMC3381310
- DOI: 10.1113/jphysiol.2011.220236
Phosphatase and tensin homologue (PTEN) regulates synaptic plasticity independently of its effect on neuronal morphology and migration
Abstract
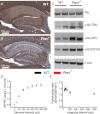
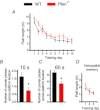
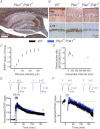
The tumour suppressor PTEN is the central negative regulator of the phosphatidylinositol 3-kinase (PI3K) signalling pathway, which mediates diverse processes in various tissues. In the nervous system, the PI3K pathway modulates proliferation, migration, cellular size, synaptic transmission and plasticity. In humans, neurological abnormalities such as autism, seizures and ataxia are associated with inherited PTEN mutations. In rodents, Pten loss during early development is associated with extensive deficits in neuronal migration and substantial hypertrophy of neurons and synaptic densities; however, whether its effect on synaptic transmission and plasticity is direct or mediated by structural abnormalities remains unknown. Here we analysed neuronal and synaptic structures and function in Pten-conditional knockout mice in which the gene was deleted from excitatory neurons postnatally. Using two-photon imaging, Golgi staining, immunohistochemistry, electron microscopy, and electrophysiological tools, we determined that Pten loss does not affect hippocampus development, neuronal or synaptic structures, or basal excitatory synaptic transmission. However, it does cause deficits in both major forms of synaptic plasticity, long-term potentiation and long-term depression, of excitatory synaptic transmission. These deficits coincided with impaired spatial memory, as measured in water maze tasks. Deletion of Pdk1, which encodes a positive downstream regulator of the PI3K pathway, rescued Pten-mediated deficits in synaptic plasticity but not in spatial memory. These results suggest that PTEN independently modulates functional and structural properties of hippocampal neurons and is directly involved in mechanisms of synaptic plasticity.
Figures




Comment in
-
PTEN: a new player controlling structural and functional synaptic plasticity.J Physiol. 2012 Mar 1;590(5):1017. doi: 10.1113/jphysiol.2012.227868. J Physiol. 2012. PMID: 22399818 Free PMC article. No abstract available.
References
-
- Backman S, Stambolic V, Mak T. PTEN function in mammalian cell size regulation. Curr Opin Neurobiol. 2002;12:516–522. - PubMed
-
- Backman SA, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J, Tsao MS, Shannon P, Bolon B, Ivy GO, Mak TW. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet. 2001;29:396–403. - PubMed
-
- Bangash MA, Park JM, Melnikova T, Wang D, Jeon SK, Lee D, Syeda S, Kim J, Kouser M, Schwartz J, Cui Y, Zhao X, Speed HE, Kee SE, Tu JC, Hu JH, Petralia RS, Linden DJ, Powell CM, Savonenko A, Xiao B, Worley PF. Enhanced polyubiquitination of Shank3 and NMDA receptor in a mouse model of autism. Cell. 2011;145:758–772. - PMC - PubMed
-
- Bayascas JR, Leslie NR, Parsons R, Fleming S, Alessi DR. Hypomorphic mutation of PDK1 suppresses tumorigenesis in PTEN(+/–) mice. Curr Biol. 2005;15:1839–1846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

