The Arabidopsis tail-anchored protein PEROXISOMAL AND MITOCHONDRIAL DIVISION FACTOR1 is involved in the morphogenesis and proliferation of peroxisomes and mitochondria
- PMID: 22147290
- PMCID: PMC3269876
- DOI: 10.1105/tpc.111.090142
The Arabidopsis tail-anchored protein PEROXISOMAL AND MITOCHONDRIAL DIVISION FACTOR1 is involved in the morphogenesis and proliferation of peroxisomes and mitochondria
Abstract
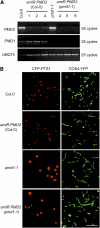
Peroxisomes and mitochondria are multifunctional eukaryotic organelles that are not only interconnected metabolically but also share proteins in division. Two evolutionarily conserved division factors, dynamin-related protein (DRP) and its organelle anchor FISSION1 (FIS1), mediate the fission of both peroxisomes and mitochondria. Here, we identified and characterized a plant-specific protein shared by these two types of organelles. The Arabidopsis thaliana PEROXISOMAL and MITOCHONDRIAL DIVISION FACTOR1 (PMD1) is a coiled-coil protein tethered to the membranes of peroxisomes and mitochondria by its C terminus. Null mutants of PMD1 contain enlarged peroxisomes and elongated mitochondria, and plants overexpressing PMD1 have an increased number of these organelles that are smaller in size and often aggregated. PMD1 lacks physical interaction with the known division proteins DRP3 and FIS1; it is also not required for DRP3's organelle targeting. Affinity purifications pulled down PMD1's homolog, PMD2, which exclusively targets to mitochondria and plays a specific role in mitochondrial morphogenesis. PMD1 and PMD2 can form homo- and heterocomplexes. Organelle targeting signals reside in the C termini of these proteins. Our results suggest that PMD1 facilitates peroxisomal and mitochondrial proliferation in a FIS1/DRP3-independent manner and that the homologous proteins PMD1 and PMD2 perform nonredundant functions in organelle morphogenesis.
Figures





References
-
- Abell B.M., Mullen R.T. (2011). Tail-anchored membrane proteins: exploring the complex diversity of tail-anchored-protein targeting in plant cells. Plant Cell Rep. 30: 137–151 - PubMed
-
- Andrade-Navarro M.A., Sanchez-Pulido L., McBride H.M. (2009). Mitochondrial vesicles: An ancient process providing new links to peroxisomes. Curr. Opin. Cell Biol. 21: 560–567 - PubMed
-
- Arimura S., Aida G.P., Fujimoto M., Nakazono M., Tsutsumi N. (2004). Arabidopsis dynamin-like protein 2a (ADL2a), like ADL2b, is involved in plant mitochondrial division. Plant Cell Physiol. 45: 236–242 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

