A randomized controlled trial of rituximab following failure of antiviral therapy for hepatitis C virus-associated cryoglobulinemic vasculitis
- PMID: 22147444
- PMCID: PMC3243106
- DOI: 10.1002/art.34322
A randomized controlled trial of rituximab following failure of antiviral therapy for hepatitis C virus-associated cryoglobulinemic vasculitis
Abstract
Objective: To perform a randomized controlled trial of rituximab in patients with hepatitis C virus (HCV)-associated mixed cryoglobulinemic vasculitis.
Methods: We conducted a single-center, open-label, randomized controlled trial of rituximab (375 mg/ m(2) /week for 4 weeks) compared to the best available therapy (maintenance or increase in immunosuppressive therapy) for HCV-associated cryoglobulinemic vasculitis in patients in whom antiviral therapy had failed to induce remission. The primary end point was disease remission at 6 months from study entry.
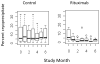
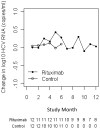
Results: A total of 24 patients were enrolled (12 in each treatment group). Baseline disease activity and organ involvement were similar in the two groups. Ten patients in the rituximab group (83%) were in remission at study month 6, as compared with 1 patient in the control group (8%), a result that met the criterion for stopping the study (P < 0.001). The median duration of remission for rituximab-treated patients who reached the primary end point was 7 months. No adverse effects of rituximab on HCV plasma viremia or on hepatic transaminase levels were observed.
Conclusion: Rituximab was a well-tolerated and effective treatment in patients with HCV-associated cryoglobulinemic vasculitis in whom antiviral therapy failed to induce remission.
Trial registration: ClinicalTrials.gov NCT00029107.
Copyright © 2012 by the American College of Rheumatology.
Figures

Comment in
-
Hepatitis C virus-related cryoglobulinemic vasculitis: emerging trends in therapy.Arthritis Rheum. 2012 Mar;64(3):604-8. doi: 10.1002/art.34326. Arthritis Rheum. 2012. PMID: 22147537 No abstract available.
-
Update in rheumatology: evidence published in 2012.Ann Intern Med. 2013 Jun 18;158(12):903-6. doi: 10.7326/0003-4819-158-12-201306180-00107. Ann Intern Med. 2013. PMID: 23580081 No abstract available.
References
-
- Galossi A, Guarisco R, Bellis L, Puoti C. Extrahepatic manifestations of chronic HCV infection. J Gastrointestin Liver Dis. 2007;16(1):65–73. - PubMed
-
- Dustin LB, Rice CM. Flying under the radar: the immunobiology of hepatitis C. Annu Rev Immunol. 2007;25:71–99. - PubMed
-
- Agnello V. The etiology and pathophysiology of mixed cryoglobulinemia secondary to hepatitis C virus infection. Springer Semin Immunopathol. 1997;19(1):111–29. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

