Comparative studies of thermotolerance: different modes of heat acclimation between tolerant and intolerant aquatic plants of the genus Potamogeton
- PMID: 22147547
- PMCID: PMC3268545
- DOI: 10.1093/aob/mcr300
Comparative studies of thermotolerance: different modes of heat acclimation between tolerant and intolerant aquatic plants of the genus Potamogeton
Abstract
Background and aims: Molecular-based studies of thermotolerance have rarely been performed on wild plants, although this trait is critical for summer survival. Here, we focused on thermotolerance and expression of heat shock transcription factor A2 (HSFA2) and its putative target gene (chloroplast-localized small heat shock protein, CP-sHSP) in two allied aquatic species of the genus Potamogeton (pondweeds) that differ in survival on land.
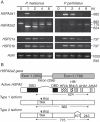
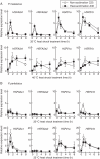
Methods: The degree of thermotolerance was examined using a chlorophyll bioassay to assess heat injury in plants cultivated under non- and heat-acclimation conditions. Potamogeton HSFA2 and CP-sHSP genes were identified and their heat-induction was quantified by real-time PCR.
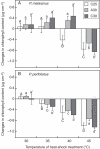
Key results: The inhibition of chlorophyll accumulation after heat stress showed that Potamogeton malaianus had a higher basal thermotolerance and developed acquired thermotolerance, whereas Potamogeton perfoliatus was heat sensitive and unable to acquire thermotolerance. We found two duplicated HSFA2 and CP-sHSP genes in each species. These genes were induced by heat shock in P. malaianus, while one HSFA2a gene was not induced in P. perfoliatus. In non-heat-acclimated plants, transcript levels of HSFA2 and CP-sHSP were transiently elevated after heat shock. In heat-acclimated plants, transcripts were continuously induced during sublethal heat shock in P. malaianus, but not in P. perfoliatus. Instead, the minimum threshold temperature for heat induction of the CP-sHSP genes was elevated in P. perfoliatus.
Conclusions: Our comparative study of thermotolerance showed that heat acclimation leads to species-specific changes in heat response. The development of acquired thermotolerance is beneficial for survival at extreme temperatures. However, the loss of acquired thermotolerance and plasticity in the minimum threshold temperature of heat response may be favourable for plants growing in moderate habitats with limited daily and seasonal temperature fluctuations.
Figures


Similar articles
-
Analysis of gene sequences indicates that quantity not quality of chloroplast small HSPs improves thermotolerance in C4 and CAM plants.Plant Cell Rep. 2012 Oct;31(10):1943-57. doi: 10.1007/s00299-012-1307-z. Epub 2012 Jul 14. Plant Cell Rep. 2012. PMID: 22797908
-
HsfA2 Controls the Activity of Developmentally and Stress-Regulated Heat Stress Protection Mechanisms in Tomato Male Reproductive Tissues.Plant Physiol. 2016 Apr;170(4):2461-77. doi: 10.1104/pp.15.01913. Epub 2016 Feb 25. Plant Physiol. 2016. PMID: 26917685 Free PMC article.
-
Ecotypic variation in chloroplast small heat-shock proteins and related thermotolerance in Chenopodium album.Plant Physiol Biochem. 2011 Aug;49(8):898-908. doi: 10.1016/j.plaphy.2011.05.002. Epub 2011 May 23. Plant Physiol Biochem. 2011. PMID: 21684754
-
Heat Stress Responses and Thermotolerance in Maize.Int J Mol Sci. 2021 Jan 19;22(2):948. doi: 10.3390/ijms22020948. Int J Mol Sci. 2021. PMID: 33477941 Free PMC article. Review.
-
HSP101: a key component for the acquisition of thermotolerance in plants.Plant Cell. 2000 Apr;12(4):457-60. doi: 10.1105/tpc.12.4.457. Plant Cell. 2000. PMID: 10760235 Free PMC article. Review. No abstract available.
Cited by
-
Regulation of alternative splicing in response to temperature variation in plants.J Exp Bot. 2021 Sep 30;72(18):6150-6163. doi: 10.1093/jxb/erab232. J Exp Bot. 2021. PMID: 34028544 Free PMC article. Review.
-
Integrating Omics and Alternative Splicing Reveals Insights into Grape Response to High Temperature.Plant Physiol. 2017 Feb;173(2):1502-1518. doi: 10.1104/pp.16.01305. Epub 2017 Jan 3. Plant Physiol. 2017. PMID: 28049741 Free PMC article.
-
An autoregulatory loop controlling Arabidopsis HsfA2 expression: role of heat shock-induced alternative splicing.Plant Physiol. 2013 May;162(1):512-21. doi: 10.1104/pp.112.205864. Epub 2013 Mar 15. Plant Physiol. 2013. PMID: 23503691 Free PMC article.
-
Boechera species exhibit species-specific responses to combined heat and high light stress.PLoS One. 2015 Jun 1;10(6):e0129041. doi: 10.1371/journal.pone.0129041. eCollection 2015. PLoS One. 2015. PMID: 26030823 Free PMC article.
-
A review of changes at the phenotypic, physiological, biochemical, and molecular levels of plants due to high temperatures.Planta. 2024 Feb 3;259(3):57. doi: 10.1007/s00425-023-04320-y. Planta. 2024. PMID: 38307982 Review.
References
-
- Baniwal SK, Bharti K, Chan KY, et al. Heat stress response in plants: a complex game with chaperones and more than twenty heat stress transcription factors. Journal of Biosciences. 2004;29:471–487. - PubMed
-
- Barua D, Heckathorn SA. Acclimation of the temperature set-points of the heat-shock response. Journal of Thermal Biology. 2004;29:185–193.
-
- Barua D, Heckathorn SA, Downs CA. Variation in chloroplast small heat-shock protein function is a major determinant of variation in thermotolerance of photosynthetic electron transport among ecotypes of Chenopodium album. Functional Plant Biology. 2003;30:1071–1079. - PubMed
-
- Berry J, Björkman O. Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology. 1980;31:491–543.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

