The skeletal muscle satellite cell: still young and fascinating at 50
- PMID: 22147605
- PMCID: PMC3283088
- DOI: 10.1369/0022155411426780
The skeletal muscle satellite cell: still young and fascinating at 50
Abstract
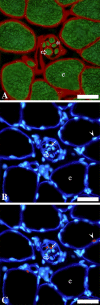
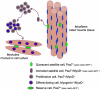
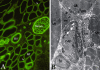
The skeletal muscle satellite cell was first described and named based on its anatomic location between the myofiber plasma and basement membranes. In 1961, two independent studies by Alexander Mauro and Bernard Katz provided the first electron microscopic descriptions of satellite cells in frog and rat muscles. These cells were soon detected in other vertebrates and acquired candidacy as the source of myogenic cells needed for myofiber growth and repair throughout life. Cultures of isolated myofibers and, subsequently, transplantation of single myofibers demonstrated that satellite cells were myogenic progenitors. More recently, satellite cells were redefined as myogenic stem cells given their ability to self-renew in addition to producing differentiated progeny. Identification of distinctively expressed molecular markers, in particular Pax7, has facilitated detection of satellite cells using light microscopy. Notwithstanding the remarkable progress made since the discovery of satellite cells, researchers have looked for alternative cells with myogenic capacity that can potentially be used for whole body cell-based therapy of skeletal muscle. Yet, new studies show that inducible ablation of satellite cells in adult muscle impairs myofiber regeneration. Thus, on the 50th anniversary since its discovery, the satellite cell's indispensable role in muscle repair has been reaffirmed.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the authorship and publication of this article.
Figures


References
-
- Aguennouz M, Vita GL, Messina S, Cama A, Lanzano N, Ciranni A, Rodolico C, Di Giorgio RM, Vita G. 2011. Telomere shortening is associated to TRF1 and PARP1 overexpression in Duchenne muscular dystrophy. Neurobiol Aging. 32:2190–2197 - PubMed
-
- Anderson JE. 2006. The satellite cell as a companion in skeletal muscle plasticity: currency, conveyance, clue, connector and colander. J Exp Biol. 209:2276–2292 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

