Chaperoning of mutant p53 protein by wild-type p53 protein causes hypoxic tumor regression
- PMID: 22147694
- PMCID: PMC3268447
- DOI: 10.1074/jbc.M111.317354
Chaperoning of mutant p53 protein by wild-type p53 protein causes hypoxic tumor regression
Abstract
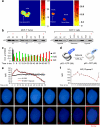
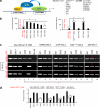
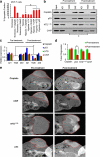
Mutant (Mt) p53 abrogates tumor suppression functions of wild-type (WT) p53 through mutant-specific, gain-of-function effects, and patients bearing Mt p53 are chemoresistant. The dominant negative effect of p53 mutants results from their aggregation propensity which causes co-aggregation of WT p53. We explored the mechanism of p53 inactivation in hypoxia and hypothesized whether WT p53 could rescue Mt p53 in hypoxic tumors. WT p53 exists in mutant conformation in hypoxic core of MCF-7 solid tumors, and its conformation is oxygen-dependent. Under simulated hypoxia in cells, WT p53 undergoes conformational change in acquiring mutant conformation. An in vivo chaperone assay shows that WT p53 functions as a molecular chaperone in rescuing conformational and structural p53 mutants in cancer cells both at the transcription and proteome levels. WT p53 chaperone therapy is further shown to cause significant regression of tumor xenografts through reconversion of the mutant phenotype to wild-type p53. The chaperone function of WT p53 is directly linked to the induction of apoptosis in both cancer cells and tumor xenografts. As oncogenic p53 mutants are linked to chemoresistance in hypoxic tumors, p53 chaperone therapy will introduce new dimensions to existing cancer therapeutics. We propose that in cancer cells, WT p53 chaperoning may either exist as a cellular event to potentially reverse the dominant negative effect of its oncogenic mutants or to stabilize yet unidentified factors.
Figures

Similar articles
-
Re-oxygenation causes hypoxic tumor regression through restoration of p53 wild-type conformation and post-translational modifications.Cell Death Dis. 2012 Mar 15;3(3):e286. doi: 10.1038/cddis.2012.15. Cell Death Dis. 2012. PMID: 22419115 Free PMC article.
-
Gain of function of mutant p53 by coaggregation with multiple tumor suppressors.Nat Chem Biol. 2011 May;7(5):285-95. doi: 10.1038/nchembio.546. Epub 2011 Mar 27. Nat Chem Biol. 2011. PMID: 21445056
-
p53 from complexity to simplicity: mutant p53 stabilization, gain-of-function, and dominant-negative effect.FASEB J. 2000 Oct;14(13):1901-7. doi: 10.1096/fj.99-1078rev. FASEB J. 2000. PMID: 11023974 Review.
-
Hypoxia and hypoxia mimetic cooperate to counteract tumor cell resistance to glucose starvation preferentially in tumor cells with mutant p53.Biochem Biophys Res Commun. 2014 Jan 3;443(1):120-5. doi: 10.1016/j.bbrc.2013.11.065. Epub 2013 Nov 22. Biochem Biophys Res Commun. 2014. PMID: 24275138
-
Chaperoning the guardian of the genome. The two-faced role of molecular chaperones in p53 tumor suppressor action.Biochim Biophys Acta Rev Cancer. 2018 Apr;1869(2):161-174. doi: 10.1016/j.bbcan.2017.12.004. Epub 2018 Jan 31. Biochim Biophys Acta Rev Cancer. 2018. PMID: 29355591 Review.
Cited by
-
Regulation of glucose metabolism by p53: emerging new roles for the tumor suppressor.Oncotarget. 2011 Dec;2(12):948-57. doi: 10.18632/oncotarget.389. Oncotarget. 2011. PMID: 22248668 Free PMC article. Review.
-
The curcumin analog HO-3867 selectively kills cancer cells by converting mutant p53 protein to transcriptionally active wildtype p53.J Biol Chem. 2018 Mar 23;293(12):4262-4276. doi: 10.1074/jbc.RA117.000950. Epub 2018 Jan 30. J Biol Chem. 2018. PMID: 29382728 Free PMC article.
-
Mutant p53 in cancer: new functions and therapeutic opportunities.Cancer Cell. 2014 Mar 17;25(3):304-17. doi: 10.1016/j.ccr.2014.01.021. Cancer Cell. 2014. PMID: 24651012 Free PMC article. Review.
-
p53's choice of myocardial death or survival: Oxygen protects infarct myocardium by recruiting p53 on NOS3 promoter through regulation of p53-Lys(118) acetylation.EMBO Mol Med. 2013 Nov;5(11):1662-83. doi: 10.1002/emmm.201202055. Epub 2013 Oct 1. EMBO Mol Med. 2013. PMID: 24096875 Free PMC article.
-
Tumorigenic p53 mutants undergo common structural disruptions including conversion to α-sheet structure.Protein Sci. 2020 Sep;29(9):1983-1999. doi: 10.1002/pro.3921. Epub 2020 Aug 17. Protein Sci. 2020. PMID: 32715544 Free PMC article.
References
-
- Schnitzer S. E., Schmid T., Zhou J., Brüne B. (2006) Hypoxia and HIF-1α protect A549 cells from drug-induced apoptosis. Cell Death Differ. 13, 1611–1613 - PubMed
-
- Achison M., Hupp T. R. (2003) Hypoxia attenuates the p53 response to cellular damage. Oncogene 22, 3431–3440 - PubMed
-
- Hammond E. M., Giaccia A. J. (2006) Hypoxia-inducible factor-1 and p53: friends, acquaintances, or strangers? Clin. Cancer Res. 12, 5007–5009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

