Redox outside the box: linking extracellular redox remodeling with intracellular redox metabolism
- PMID: 22147695
- PMCID: PMC3281658
- DOI: 10.1074/jbc.R111.287995
Redox outside the box: linking extracellular redox remodeling with intracellular redox metabolism
Abstract
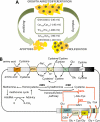
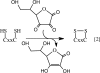
Aerobic organisms generate reactive oxygen species as metabolic side products and must achieve a delicate balance between using them for signaling cellular functions and protecting against collateral damage. Small molecule (e.g. glutathione and cysteine)- and protein (e.g. thioredoxin)-based buffers regulate the ambient redox potentials in the various intracellular compartments, influence the status of redox-sensitive macromolecules, and protect against oxidative stress. Less well appreciated is the fact that the redox potential of the extracellular compartment is also carefully regulated and is dynamic. Changes in intracellular metabolism alter the redox poise in the extracellular compartment, and these are correlated with cellular processes such as proliferation, differentiation, and death. In this minireview, the mechanism of extracellular redox remodeling due to intracellular sulfur metabolism is discussed in the context of various cell-cell communication paradigms.
Figures

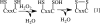
Similar articles
-
Extracellular redox state: refining the definition of oxidative stress in aging.Rejuvenation Res. 2006 Summer;9(2):169-81. doi: 10.1089/rej.2006.9.169. Rejuvenation Res. 2006. PMID: 16706639 Review.
-
Spatio-temporal changes in glutathione and thioredoxin redox couples during ionizing radiation-induced oxidative stress regulate tumor radio-resistance.Free Radic Res. 2015 Oct;49(10):1218-32. doi: 10.3109/10715762.2015.1056180. Free Radic Res. 2015. PMID: 26021764
-
Role of redox potential and reactive oxygen species in stress signaling.Oncogene. 1999 Nov 1;18(45):6104-11. doi: 10.1038/sj.onc.1203128. Oncogene. 1999. PMID: 10557101 Review.
-
Increased reactive oxygen species production during reductive stress: The roles of mitochondrial glutathione and thioredoxin reductases.Biochim Biophys Acta. 2015 Jun-Jul;1847(6-7):514-25. doi: 10.1016/j.bbabio.2015.02.012. Epub 2015 Feb 19. Biochim Biophys Acta. 2015. PMID: 25701705 Free PMC article.
-
Oxidative Modification of Redox Proteins: Role in the Regulation of HBL-100 Cell Proliferation.Bull Exp Biol Med. 2019 May;167(1):30-34. doi: 10.1007/s10517-019-04453-9. Epub 2019 Jun 8. Bull Exp Biol Med. 2019. PMID: 31177465
Cited by
-
New Titanocene (IV) Dicarboxylates with Potential Cytotoxicity: Synthesis, Structure, Stability and Electrochemistry.Int J Mol Sci. 2023 Feb 7;24(4):3340. doi: 10.3390/ijms24043340. Int J Mol Sci. 2023. PMID: 36834751 Free PMC article.
-
Micronutrients in Support to The Carbon Cycle Activate Antioxidant Defences and Reduce Sperm DNA Damage in Infertile Men Attending Assisted Reproductive Technology Programs: Clinical Trial Study.Int J Fertil Steril. 2020 Apr;14(1):57-62. doi: 10.22074/ijfs.2020.6084. Epub 2020 Feb 25. Int J Fertil Steril. 2020. PMID: 32112637 Free PMC article.
-
Extracellular Redox Regulation of Intracellular Reactive Oxygen Generation, Mitochondrial Function and Lipid Turnover in Cultured Human Adipocytes.PLoS One. 2016 Oct 14;11(10):e0164011. doi: 10.1371/journal.pone.0164011. eCollection 2016. PLoS One. 2016. PMID: 27741233 Free PMC article.
-
Protein Classes Predicted by Molecular Surface Chemical Features: Machine Learning-Assisted Classification of Cytosol and Secreted Proteins.J Phys Chem B. 2024 Sep 5;128(35):8423-8436. doi: 10.1021/acs.jpcb.4c02461. Epub 2024 Aug 26. J Phys Chem B. 2024. PMID: 39185763 Free PMC article.
-
The thioredoxin system in breast cancer cell invasion and migration.Redox Biol. 2016 Aug;8:68-78. doi: 10.1016/j.redox.2015.12.004. Epub 2015 Dec 19. Redox Biol. 2016. PMID: 26760912 Free PMC article.
References
-
- Irani K., Xia Y., Zweier J. L., Sollott S. J., Der C. J., Fearon E. R., Sundaresan M., Finkel T., Goldschmidt-Clermont P. J. (1997) Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 275, 1649–1652 - PubMed
-
- Bae Y. S., Kang S. W., Seo M. S., Baines I. C., Tekle E., Chock P. B., Rhee S. G. (1997) Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 272, 217–221 - PubMed
-
- Franco R., Schoneveld O. J., Pappa A., Panayiotidis M. I. (2007) The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 113, 234–258 - PubMed
-
- Moriarty-Craige S. E., Jones D. P. (2004) Extracellular thiols and thiol/disulfide redox in metabolism. Annu. Rev. Nutr. 24, 481–509 - PubMed
-
- Weissbach H., Resnick L., Brot N. (2005) Methionine sulfoxide reductases: history and cellular role in protecting against oxidative damage. Biochim. Biophys. Acta 1703, 203–212 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

