Calcium binding to leptospira outer membrane antigen LipL32 is not necessary for its interaction with plasma fibronectin, collagen type IV, and plasminogen
- PMID: 22147698
- PMCID: PMC3281616
- DOI: 10.1074/jbc.M111.277210
Calcium binding to leptospira outer membrane antigen LipL32 is not necessary for its interaction with plasma fibronectin, collagen type IV, and plasminogen
Abstract
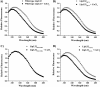
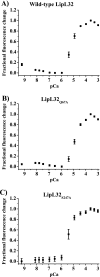
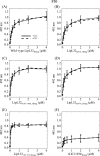
LipL32 is the most abundant outer membrane protein from pathogenic Leptospira and has been shown to bind extracellular matrix (ECM) proteins as well as Ca(2+). Recent crystal structures have been obtained for the protein in the apo- and Ca(2+)-bound forms. In this work, we produced three LipL32 mutants (D163-168A, Q67A, and S247A) and evaluated their ability to interact with Ca(2+) and with ECM glycoproteins and human plasminogen. The D163-168A mutant modifies aspartate residues involved in Ca(2+) binding, whereas the other two modify residues in a cavity on the other side of the protein structure. Loss of calcium binding in the D163-D168A mutant was confirmed using intrinsic tryptophan fluorescence, circular dichroism, and thermal denaturation whereas the Q67A and S247A mutants presented the same Ca(2+) affinity as the wild-type protein. We then evaluated if Ca(2+) binding to LipL32 would be crucial for its interaction with collagen type IV and plasma proteins fibronectin and plasminogen. Surprisingly, the wild-type protein and all three mutants, including the D163-168A variant, bound to these ECM proteins with very similar affinities, both in the presence and absence of Ca(2+) ions. In conclusion, calcium binding to LipL32 may be important to stabilize the protein, but is not necessary to mediate interaction with host extracellular matrix proteins.
Figures
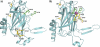


References
-
- Faine S., Adler B., Bolin C., Perolat P. (1999) Leptospira and Leptospirosis, 2nd Ed., MedSci, Melbourne, Australia
-
- Adler B., de la Peña Moctezuma A. (2010) Leptospira and leptospirosis. Vet. Microbiol. 140, 287–296 - PubMed
-
- Picardeau M., Bulach D. M., Bouchier C., Zuerner R. L., Zidane N., Wilson P. J., Creno S., Kuczek E. S., Bommezzadri S., Davis J. C., McGrath A., Johnson M. J., Boursaux-Eude C., Seemann T., Rouy Z., Coppel R. L., Rood J. I., Lajus A., Davies J. K., Médigue C., Adler B. (2008) Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS One 3, e1607. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

