Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides
- PMID: 22147704
- PMCID: PMC3281607
- DOI: 10.1074/jbc.R111.283432
Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides
Abstract
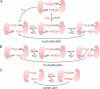
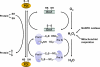
Peroxiredoxins (Prxs) contain an active site cysteine that is sensitive to oxidation by H(2)O(2). Mammalian cells express six Prx isoforms that are localized to various cellular compartments. The oxidized active site cysteine of Prx can be reduced by a cellular thiol, thus enabling Prx to function as a locally constrained peroxidase. Regulation of Prx via phosphorylation in response to extracellular signals allows the local accumulation of H(2)O(2) and thereby enables its messenger function. The fact that the oxidation state of the active site cysteine of Prx can be transferred to other proteins that are less intrinsically susceptible to H(2)O(2) also allows Prx to function as an H(2)O(2) sensor.
Figures


References
-
- Kim K., Kim I. H., Lee K. Y., Rhee S. G., Stadtman E. R. (1988) The isolation and purification of a specific “protector” protein which inhibits enzyme inactivation by a thiol/Fe(III)/O2 mixed-function oxidation system. J. Biol. Chem. 263, 4704–4711 - PubMed
-
- Chae H. Z., Kim I. H., Kim K., Rhee S. G. (1993) Cloning, sequencing, and mutation of thiol-specific antioxidant gene of Saccharomyces cerevisiae. J. Biol. Chem. 268, 16815–16821 - PubMed
-
- Chae H. Z., Robison K., Poole L. B., Church G., Storz G., Rhee S. G. (1994) Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc. Natl. Acad. Sci. U.S.A. 91, 7017–7021 - PMC - PubMed
-
- Rhee S. G., Woo H. A. (2011) Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H2O2, and protein chaperones. Antioxid. Redox Signal. 15, 781–794 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

