Structural and functional insights into the DNA replication factor Cdc45 reveal an evolutionary relationship to the DHH family of phosphoesterases
- PMID: 22147708
- PMCID: PMC3281742
- DOI: 10.1074/jbc.M111.285395
Structural and functional insights into the DNA replication factor Cdc45 reveal an evolutionary relationship to the DHH family of phosphoesterases
Abstract
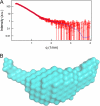
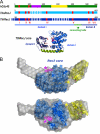
Cdc45 is an essential protein conserved in all eukaryotes and is involved both in the initiation of DNA replication and the progression of the replication fork. With GINS, Cdc45 is an essential cofactor of the Mcm2-7 replicative helicase complex. Despite its importance, no detailed information is available on either the structure or the biochemistry of the protein. Intriguingly, whereas homologues of both GINS and Mcm proteins have been described in Archaea, no counterpart for Cdc45 is known. Herein we report a bioinformatic analysis that shows a weak but significant relationship among eukaryotic Cdc45 proteins and a large family of phosphoesterases that has been described as the DHH family, including inorganic pyrophosphatases and RecJ ssDNA exonucleases. These enzymes catalyze the hydrolysis of phosphodiester bonds via a mechanism involving two Mn(2+) ions. Only a subset of the amino acids that coordinates Mn(2+) is conserved in Cdc45. We report biochemical and structural data on the recombinant human Cdc45 protein, consistent with the proposed DHH family affiliation. Like the RecJ exonucleases, the human Cdc45 protein is able to bind single-stranded, but not double-stranded DNA. Small angle x-ray scattering data are consistent with a model compatible with the crystallographic structure of the RecJ/DHH family members.
Figures


References
-
- Aparicio O. M., Weinstein D. M., Bell S. P. (1997) Components and dynamics of DNA replication complexes in S. cerevisiae. Redistribution of MCM proteins and Cdc45p during S phase. Cell 91, 59–69 - PubMed
-
- Uchiyama M., Arai K., Masai H. (2001) Sna41goa1, a novel mutation causing G1/S arrest in fission yeast, is defective in a CDC45 homolog and interacts genetically with polα. Mol. Genet. Genomics 265, 1039–1049 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

