Immunization of mice with vibrio cholerae outer-membrane vesicles protects against hyperinfectious challenge and blocks transmission
- PMID: 22147790
- PMCID: PMC3256948
- DOI: 10.1093/infdis/jir756
Immunization of mice with vibrio cholerae outer-membrane vesicles protects against hyperinfectious challenge and blocks transmission
Abstract
Background: Vibrio cholerae excreted by cholera patients is "hyperinfectious" (HI), which can be modeled by passage through infant mice. Immunization of adult female mice with V. cholerae outer-membrane vesicles (OMVs) passively protects suckling mice from challenge. Although V. cholerae is unable to colonize protected pups, the bacteria survive passage and have the potential to be transmitted to susceptible individuals. Here, we investigated the impact of OMV immunization and the HI state on V. cholerae transmission.
Methods: Neonatal mice suckled by OMV- or sham-immunized dams were challenged with HI V. cholerae. The infectivity of spatially and temporally separate V. cholerae populations obtained from infected naive or protected pups was tested. Recombination-based in vivo expression technology was used to assess virulence gene expression within these populations.
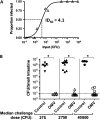
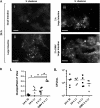
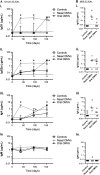
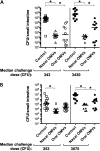
Results: OMV immunization significantly reduced colonization of neonates challenged with HI V. cholerae. Vibrio cholerae that had colonized the naive host was HI, whereas V. cholerae excreted by neonates born to OMV-immunized dams, although viable, was hypoinfectious and failed to fully induce virulence gene expression.
Conclusions: OMV immunization can significantly reduce the V. cholerae burden upon challenge with HI V. cholerae and can also block transmission from immune mice by reducing the infectivity of shed bacteria.
Figures


Similar articles
-
Characterization of Vibrio cholerae outer membrane vesicles as a candidate vaccine for cholera.Infect Immun. 2009 Jan;77(1):472-84. doi: 10.1128/IAI.01139-08. Epub 2008 Nov 10. Infect Immun. 2009. PMID: 19001078 Free PMC article.
-
Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice.Infect Immun. 2008 Oct;76(10):4554-63. doi: 10.1128/IAI.00532-08. Epub 2008 Aug 4. Infect Immun. 2008. PMID: 18678672 Free PMC article.
-
Mucosal immunization with Vibrio cholerae outer membrane vesicles provides maternal protection mediated by antilipopolysaccharide antibodies that inhibit bacterial motility.Infect Immun. 2010 Oct;78(10):4402-20. doi: 10.1128/IAI.00398-10. Epub 2010 Aug 2. Infect Immun. 2010. PMID: 20679439 Free PMC article.
-
Exploiting cholera vaccines as a versatile antigen delivery platform.Biotechnol Lett. 2008 Apr;30(4):571-9. doi: 10.1007/s10529-007-9594-0. Epub 2007 Nov 16. Biotechnol Lett. 2008. PMID: 18008168 Free PMC article. Review.
-
Vibrio cholerae and cholera: out of the water and into the host.FEMS Microbiol Rev. 2002 Jun;26(2):125-39. doi: 10.1111/j.1574-6976.2002.tb00605.x. FEMS Microbiol Rev. 2002. PMID: 12069878 Review.
Cited by
-
Production of outer membrane vesicles and outer membrane tubes by Francisella novicida.J Bacteriol. 2013 Mar;195(6):1120-32. doi: 10.1128/JB.02007-12. Epub 2012 Dec 21. J Bacteriol. 2013. PMID: 23264574 Free PMC article.
-
Immunogenic Properties of MVs Containing Structural Hantaviral Proteins: An Original Study.Pharmaceutics. 2022 Jan 1;14(1):93. doi: 10.3390/pharmaceutics14010093. Pharmaceutics. 2022. PMID: 35056989 Free PMC article.
-
Vibrio cholerae-induced inflammation in the neonatal mouse cholera model.Infect Immun. 2014 Jun;82(6):2434-47. doi: 10.1128/IAI.00054-14. Epub 2014 Mar 31. Infect Immun. 2014. PMID: 24686062 Free PMC article.
-
Histophilus somni causes extracellular trap formation by bovine neutrophils and macrophages.Microb Pathog. 2013 Jan;54:67-75. doi: 10.1016/j.micpath.2012.09.007. Epub 2012 Sep 27. Microb Pathog. 2013. PMID: 23022668 Free PMC article.
-
Versatile effects of bacterium-released membrane vesicles on mammalian cells and infectious/inflammatory diseases.Acta Pharmacol Sin. 2018 Apr;39(4):514-533. doi: 10.1038/aps.2017.82. Epub 2017 Aug 31. Acta Pharmacol Sin. 2018. PMID: 28858295 Free PMC article. Review.
References
-
- Sack DA, Sack RB, Nair GB, Siddique AK. Cholera. Lancet. 2004;363:223–33. - PubMed
-
- Pruzzo C, Vezzulli L, Colwell RR. Global impact of Vibrio cholerae interactions with chitin. Environ Microbiol. 2008;10:1400–10. - PubMed
-
- Childers BM, Klose KE. Regulation of virulence in Vibrio cholerae: the ToxR regulon. Future Microbiol. 2007;2:335–44. - PubMed
-
- Klose KE. The suckling mouse model of cholera. Trends Microbiol. 2000;8:189–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

