Trajectories of depression severity in clinical trials of duloxetine: insights into antidepressant and placebo responses
- PMID: 22147842
- PMCID: PMC3339151
- DOI: 10.1001/archgenpsychiatry.2011.132
Trajectories of depression severity in clinical trials of duloxetine: insights into antidepressant and placebo responses
Abstract
Context: The high percentage of failed clinical trials in depression may be due to high placebo response rates and the failure of standard statistical approaches to capture heterogeneity in treatment response.
Objective: To assess whether growth mixture modeling can provide insights into antidepressant and placebo responses in clinical trials of patients with major depression.
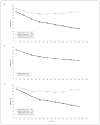
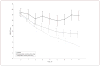
Design: We reanalyzed clinical trials of duloxetine to identify distinct trajectories of Hamilton Scale for Depression (HAM-D) scores during treatment. We analyzed the trajectories in the entire sample and then separately in all active arms and in all placebo arms. Effects of duloxetine hydrochloride, selective serotonin reuptake inhibitor (SSRI), and covariates on the probability of following a particular trajectory were assessed. Outcomes in different trajectories were compared using mixed-effects models.
Setting: Seven randomized double-blind clinical trials of duloxetine vs placebo and comparator SSRI. Patients A total of 2515 patients with major depression.
Interventions: Duloxetine and comparator SSRI. Main Outcome Measure Total score on the HAM-D.
Results: In the entire sample and in the antidepressant-treated subsample, we identified trajectories of responders (76.3% of the sample) and nonresponders (23.7% of the sample). However, placebo-treated patients were characterized by a single response trajectory. Duloxetine and SSRI did not differ in efficacy, and compared with placebo they significantly decreased the odds of following the nonresponder trajectory. Antidepressant responders had significantly better HAM-D scores over time than placebo-treated patients, but antidepressant nonresponders had significantly worse HAM-D scores over time than the placebo-treated patients.
Conclusions: Most patients treated with serotonergic antidepressants showed a clinical trajectory over time that is superior to that of placebo-treated patients. However, some patients receiving these medications did more poorly than patients receiving placebo. These data highlight the importance of ongoing monitoring of medication risks and benefits during serotonergic antidepressant treatment. They should further stimulate the search for biomarkers or other predictors of responder status in guiding antidepressant treatment.
Trial registration: ClinicalTrials.gov NCT00073411.
Figures
Similar articles
-
Duloxetine efficacy for major depressive disorder in male vs. female patients: data from 7 randomized, double-blind, placebo-controlled trials.J Clin Psychiatry. 2006 May;67(5):761-70. doi: 10.4088/jcp.v67n0510. J Clin Psychiatry. 2006. PMID: 16841626
-
Duloxetine versus escitalopram and placebo in the treatment of patients with major depressive disorder: onset of antidepressant action, a non-inferiority study.Curr Med Res Opin. 2007 Feb;23(2):401-16. doi: 10.1185/030079906X167453. Curr Med Res Opin. 2007. PMID: 17288694 Clinical Trial.
-
Trajectories of relapse in randomised, placebo-controlled trials of treatment discontinuation in major depressive disorder: an individual patient-level data meta-analysis.Lancet Psychiatry. 2017 Mar;4(3):230-237. doi: 10.1016/S2215-0366(17)30038-X. Epub 2017 Feb 9. Lancet Psychiatry. 2017. PMID: 28189575 Free PMC article. Clinical Trial.
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
-
Duloxetine: a review of its use in the treatment of generalized anxiety disorder.CNS Drugs. 2009;23(6):523-41. doi: 10.2165/00023210-200923060-00006. CNS Drugs. 2009. PMID: 19480470 Review.
Cited by
-
A model of placebo response in antidepressant clinical trials.Am J Psychiatry. 2013 Jul;170(7):723-33. doi: 10.1176/appi.ajp.2012.12040474. Am J Psychiatry. 2013. PMID: 23318413 Free PMC article. Review.
-
Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study.Mol Psychiatry. 2016 Jan;21(1):71-9. doi: 10.1038/mp.2015.22. Epub 2015 Mar 24. Mol Psychiatry. 2016. PMID: 25802980 Free PMC article. Clinical Trial.
-
Genetic variants in combination with early partial improvement as a clinical utility predictor of treatment outcome in major depressive disorder: the result of two pooled RCTs.Transl Psychiatry. 2015 Feb 24;5(2):e513. doi: 10.1038/tp.2015.6. Transl Psychiatry. 2015. PMID: 25710119 Free PMC article. Clinical Trial.
-
Optimising treatment decision rules through generated effect modifiers: a precision medicine tutorial.BJPsych Open. 2019 Dec 3;6(1):e2. doi: 10.1192/bjo.2019.85. BJPsych Open. 2019. PMID: 31791433 Free PMC article.
-
Trajectories of Symptom Change in School-Based Prevention Programs for Adolescent Girls with Subclinical Depression.J Youth Adolesc. 2022 Apr;51(4):659-672. doi: 10.1007/s10964-022-01578-5. Epub 2022 Feb 3. J Youth Adolesc. 2022. PMID: 35113294 Free PMC article. Clinical Trial.
References
-
- Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66(8):848–856. - PubMed
-
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358(3):252–260. - PubMed
-
- Wisniewski SR, Rush AJ, Nierenberg AA, Gaynes BN, Warden D, Luther JF, McGrath PJ, Lavori PW, Thase ME, Fava M, Trivedi MH. Can phase III trial results of antidepressant medications be generalized to clinical practice? A STAR*D report. Am J Psychiatry. 2009;166(5):599–607. - PubMed
-
- Stone M, Laughren T, Jones ML, Levenson M, Holland PC, Hughes A, Hammad TA, Temple R, Rochester G. Risk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US Food and Drug Administration. [Accessed. August 24, 2011];BMJ. 2009 339:b2880. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

