Erythrocyte plasma membrane-bound ERK1/2 activation promotes ICAM-4-mediated sickle red cell adhesion to endothelium
- PMID: 22147898
- PMCID: PMC3277354
- DOI: 10.1182/blood-2011-03-344440
Erythrocyte plasma membrane-bound ERK1/2 activation promotes ICAM-4-mediated sickle red cell adhesion to endothelium
Abstract
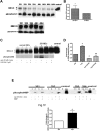
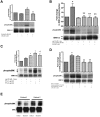
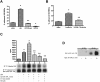
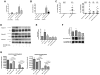
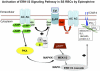
The core pathology of sickle cell disease (SCD) starts with the erythrocyte (RBC). Aberration in MAPK/ERK1/2 signaling, which can regulate cell adhesion, occurs in diverse pathologies. Because RBCs contain abundant ERK1/2, we predicted that ERK1/2 is functional in sickle (SS) RBCs and promotes adherence, a hallmark of SCD. ERK1/2 remained active in SS but not normal RBCs. β(2)-adrenergic receptor stimulation by epinephrine can enhance ERK1/2 activity only in SS RBCs via PKA- and tyrosine kinase p72(syk)-dependent pathways. ERK signaling is implicated in RBC ICAM-4 phosphorylation, promoting SS RBC adhesion to the endothelium. SS RBC adhesion and phosphorylation of both ERK and ICAM-4 all decreased with continued cell exposure to epinephrine, implying that activation of ICAM-4-mediated SS RBC adhesion is temporally associated with ERK1/2 activation. Furthermore, recombinant ERK2 phosphorylated α- and β-adducins and dematin at the ERK consensus motif. Cytoskeletal protein 4.1 also showed dynamic phosphorylation but not at the ERK consensus motif. These results demonstrate that ERK activation induces phosphorylation of cytoskeletal proteins and the adhesion molecule ICAM-4, promoting SS RBC adhesion to the endothelium. Thus, blocking RBC ERK1/2 activation, such as that promoted by catecholamine stress hormones, could ameliorate SCD pathophysiology.
Figures
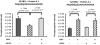
References
-
- Hebbel RP, Boogaerts MA, Eaton JW, Steinberg MH. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med. 1980;302(18):992–995. - PubMed
-
- Zennadi R, Hines PC, De Castro LM, Cartron JP, Parise LV, Telen MJ. Epinephrine acts through erythroid signaling pathways to activate sickle cell adhesion to endothelium via LW-alphavbeta3 interactions. Blood. 2004;104(12):3774–3781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

