Opposing effects of Runx2 and estradiol on breast cancer cell proliferation: in vitro identification of reciprocally regulated gene signature related to clinical letrozole responsiveness
- PMID: 22147940
- PMCID: PMC3277803
- DOI: 10.1158/1078-0432.CCR-11-1530
Opposing effects of Runx2 and estradiol on breast cancer cell proliferation: in vitro identification of reciprocally regulated gene signature related to clinical letrozole responsiveness
Abstract
Purpose: To assess the clinical significance of the interaction between estrogen and Runx2 signaling, previously shown in vitro.
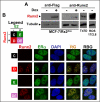
Experimental design: MCF7/Rx2(dox) breast cancer cells were treated with estradiol and/or doxycycline to induce Runx2, and global gene expression was profiled to define genes regulated by estradiol, Runx2, or both. Anchorage-independent growth was assessed by soft-agar colony formation assays. Expression of gene sets defined using the MCF7/Rx2(dox) system was analyzed in pre- and on-treatment biopsies from hormone receptor-positive patients undergoing neoadjuvant letrozole treatment in two independent studies, and short-term changes in gene expression were correlated with tumor size reduction or Ki67 index at surgery.
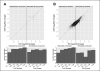
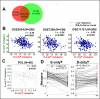
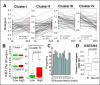
Results: Reflecting its oncogenic property, estradiol strongly promoted soft-agar colony formation, whereas Runx2 blocked this process suggesting tumor suppressor property. Transcriptome analysis of MCF7/Rx2(dox) cells treated with estradiol and/or doxycycline showed reciprocal attenuation of Runx2 and estrogen signaling. Correspondingly in breast cancer tumors, expression of estradiol- and Runx2-regulated genes was inversely correlated, and letrozole increased expression of Runx2-stimulated genes, as defined in the MCF7/Rx2(dox) model. Of particular interest was a gene set upregulated by estradiol and downregulated by Runx2 in vitro; its short-term response to letrozole treatment associated with tumor size reduction and Ki67 index at surgery better than other estradiol-regulated gene sets.
Conclusion: This work provides clinical evidence for the importance of antagonism between Runx2 and E2 signaling in breast cancer. Likely sensing the tension between them, letrozole responsiveness of a genomic node, positively regulated by estradiol and negatively regulated by Runx2 in vitro, best correlated with the clinical efficacy of letrozole treatment.
Figures


References
-
- Butt AJ, McNeil CM, Musgrove EA, Sutherland RL. Downstream targets of growth factor and oestrogen signalling and endocrine resistance: the potential roles of c-Myc, cyclin D1 and cyclin E. Endocr Relat Cancer. 2005;12(Suppl 1):S47–59. - PubMed
-
- van't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6. - PubMed
-
- Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–9. - PubMed
-
- Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst. 2006;98:262–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

