A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction
- PMID: 22147941
- PMCID: PMC3289408
- DOI: 10.1158/1078-0432.CCR-11-2121
A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction
Abstract
Purpose: Listeria monocytogenes (Lm)-based vaccines stimulate both innate and adaptive immunity. ANZ-100 is a live-attenuated Lm strain (Lm ΔactA/ΔinlB). Uptake by phagocytes in the liver results in local inflammatory responses and activation and recruitment of natural killer (NK) and T cells, in association with increased survival of mice bearing hepatic metastases. The Lm ΔactA/ΔinlB strain, engineered to express human mesothelin (CRS-207), a tumor-associated antigen expressed by a variety of tumors, induces mesothelin-specific T-cell responses against mesothelin-expressing murine tumors. These two phase I studies test ANZ-100 and CRS-207 in subjects with liver metastases and mesothelin-expressing cancers, respectively.
Experimental design: A single intravenous injection of ANZ-100 was evaluated in a dose escalation study in subjects with liver metastases. Nine subjects received 1 × 10(6), 3 × 10(7), or 3 × 10(8) colony-forming units (cfu). CRS-207 was evaluated in a dose-escalation study in subjects with mesothelioma, lung, pancreatic, or ovarian cancers. Seventeen subjects received up to 4 doses of 1 × 10(8), 3 × 10(8), 1 × 10(9), or 1 × 10(10) cfu.
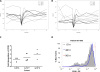
Results: A single infusion of ANZ-100 was well tolerated to the maximum planned dose. Adverse events included transient laboratory abnormalities and symptoms associated with cytokine release. Multiple infusions of CRS-207 were well tolerated up to 1 × 10(9) cfu, the determined maximum tolerated dose. Immune activation was observed for both ANZ-100 and CRS-207 as measured by serum cytokine/chemokine levels and NK cell activation. In the CRS-207 study, listeriolysin O and mesothelin-specific T-cell responses were detected and 37% of subjects lived ≥15 months.
Conclusions: ANZ-100 and CRS-207 administration was safe and resulted in immune activation.
Figures


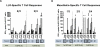
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

