Autophagy-related proteins Beclin-1 and LC3 predict cetuximab efficacy in advanced colorectal cancer
- PMID: 22147978
- PMCID: PMC3229626
- DOI: 10.3748/wjg.v17.i43.4779
Autophagy-related proteins Beclin-1 and LC3 predict cetuximab efficacy in advanced colorectal cancer
Abstract
Aim: To investigate the utility of Beclin-1 and LC3, two autophagy-related proteins, in predicting the cetuximab efficacy in advanced colorectal cancer (ACRC).
Methods: The data of 85 patients with ACRC treated at the Sun Yat-sen University Cancer Center from March 1, 2005 to December 31, 2008 were studied, including 45 cases treated with cetuximab-containing chemotherapy and 40 cases treated with non-cetuximab-containing chemotherapy. Beclin-1 and LC3 expression was evaluated by immunohistochemistry, and KRAS status was evaluated by polymerase chain reaction.
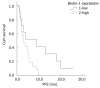
Results: Beclin-1 and LC3 expression in ACRC was significantly correlated (r = 0.44, P < 0.01); however, LC3 was more highly expressed in cancerous tissues than in normal tissues (Z = -2.63, P < 0.01). In the cetuximab-containing chemotherapy group, patients with low LC3 expression had higher objective response rates (ORRs) than those with high LC3 expression (52.9% vs 17.9%, P = 0.01), and patients with low Beclin-1 expression had a longer median progression-free survival (PFS) than their counterparts with higher Beclin-1 expression (9.0 mo vs 3.0 mo, P = 0.01). However, neither of these predictive relationships was detected in the group treated with non-cetuximab-containing chemotherapy. Patients with wild-type KRAS had higher ORRs (42.3% vs 9.1%, P = 0.049) and disease control rates (DCRs) (73.1% vs 36.4%, P = 0.035), and longer median PFS (5.5 mo vs 2.5 mo, P = 0.02) than those with mutant KRAS in the cetuximab-containing chemotherapy group. Neither Beclin-1 (P = 0.52) nor LC3 (P = 0.32) expression was significantly correlated with KRAS status.
Conclusion: Patients with low Beclin-1 expression had a longer PFS than those with high Beclin-1 expression, and patients with low LC3 expression had a higher ORR in ACRC patients treated with cetuximab-containing chemotherapy.
Keywords: Beclin-1; Cetuximab; Colorectal neoplasms; Drug therapy; LC3.
Figures


Similar articles
-
Predictive and prognostic implications of 4E-BP1, Beclin-1, and LC3 for cetuximab treatment combined with chemotherapy in advanced colorectal cancer with wild-type KRAS: Analysis from real-world data.World J Gastroenterol. 2019 Apr 21;25(15):1840-1853. doi: 10.3748/wjg.v25.i15.1840. World J Gastroenterol. 2019. PMID: 31057298 Free PMC article.
-
[Efficacy of cetuximab combined with chemotherapy for patients with advanced colorectal cancer and unclear K-ras status].Zhonghua Zhong Liu Za Zhi. 2010 Oct;32(10):777-81. Zhonghua Zhong Liu Za Zhi. 2010. PMID: 21163071 Chinese.
-
Markers for EGFR pathway activation as predictor of outcome in metastatic colorectal cancer patients treated with or without cetuximab.Eur J Cancer. 2010 Jul;46(11):1997-2009. doi: 10.1016/j.ejca.2010.03.036. Epub 2010 Apr 21. Eur J Cancer. 2010. PMID: 20413299 Clinical Trial.
-
Predictive and prognostic value of KRAS mutations in metastatic colorectal cancer patients treated with cetuximab: a meta-analysis of 22 studies.Eur J Cancer. 2010 Oct;46(15):2781-7. doi: 10.1016/j.ejca.2010.05.022. Epub 2010 Jun 25. Eur J Cancer. 2010. PMID: 20580219 Review.
-
Cetuximab: a guide to its use in combination with FOLFIRI in the first-line treatment of metastatic colorectal cancer in the USA.Mol Diagn Ther. 2012 Oct;16(5):317-22. doi: 10.1007/s40291-012-0007-2. Mol Diagn Ther. 2012. PMID: 23055389 Review.
Cited by
-
Targeting Autophagy for Overcoming Resistance to Anti-EGFR Treatments.Cancers (Basel). 2019 Sep 16;11(9):1374. doi: 10.3390/cancers11091374. Cancers (Basel). 2019. PMID: 31527477 Free PMC article. Review.
-
Prognostic impact of Beclin 1, p62/sequestosome 1 and LC3 protein expression in colon carcinomas from patients receiving 5-fluorouracil as adjuvant chemotherapy.Cancer Biol Ther. 2013 Feb;14(2):100-7. doi: 10.4161/cbt.22954. Epub 2012 Nov 28. Cancer Biol Ther. 2013. PMID: 23192274 Free PMC article.
-
Activation of endoplasmic reticulum stress promotes autophagy and apoptosis and reverses chemoresistance of human small cell lung cancer cells by inhibiting the PI3K/AKT/mTOR signaling pathway.Oncotarget. 2016 Nov 22;7(47):76827-76839. doi: 10.18632/oncotarget.12718. Oncotarget. 2016. Retraction in: Oncotarget. 2019 Jun 25;10(41):4252. doi: 10.18632/oncotarget.27064. PMID: 27765907 Free PMC article. Retracted.
-
Autophagy Modulation in Cancer: Current Knowledge on Action and Therapy.Oxid Med Cell Longev. 2018 Jan 31;2018:8023821. doi: 10.1155/2018/8023821. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29643976 Free PMC article. Review.
-
Autophagy-based survival prognosis in human colorectal carcinoma.Oncotarget. 2015 Mar 30;6(9):7084-103. doi: 10.18632/oncotarget.3054. Oncotarget. 2015. PMID: 25762626 Free PMC article.
References
-
- Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. - PubMed
-
- Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–147. - PubMed
-
- Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, McAllister PK, Morton RF, Schilsky RL. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–2096. - PubMed
-
- Röcken C. [Molecular targets for colon cancer. VEGF, EGFR - and what else?] Pathologe. 2008;29 Suppl 2:200–203. - PubMed
-
- Guo GF, Xia LP, Qiu HJ, Xu RH, Zhang B, Jiang WQ, Zhou FF, Wang F. [Efficacy of cetuximab combined with chemotherapy for patients with advanced colorectal cancer and unclear K-ras status] Zhonghua Zhong Liu Za Zhi. 2010;32:777–781. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

