Induction of apoptosis by 3-amino-6-(3-aminopropyl)-5,6-dihydro-5,11-dioxo-11H-indeno[1,2-c]isoquinoline via modulation of MAPKs (p38 and c-Jun N-terminal kinase) and c-Myc in HL-60 human leukemia cells
- PMID: 22148260
- PMCID: PMC3311722
- DOI: 10.1021/np200791j
Induction of apoptosis by 3-amino-6-(3-aminopropyl)-5,6-dihydro-5,11-dioxo-11H-indeno[1,2-c]isoquinoline via modulation of MAPKs (p38 and c-Jun N-terminal kinase) and c-Myc in HL-60 human leukemia cells
Abstract
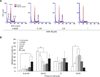
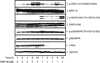
Recently, we reported that 3-amino-6-(3-aminopropyl)-5,6-dihydro-5,11-dioxo-11H-indeno[1,2-c]isoquinoline (AM6-36), sharing structural similarity with naturally occurring isoquinolines, induced activities mediated by retinoid X receptor (RXR) response element accompanied by antiproliferative effects on breast cancer cells. To further characterize the biologic potential of AM6-36, we currently report studies conducted with HL-60 human leukemia cells. AM6-36 significantly inhibited cellular proliferation in a dose- and time-dependent manner with an IC(50) value of 86 nM. When evaluated at low test concentrations (≤0.25 μM), AM6-36 induced arrest in the G2/M phase of the cell cycle. At higher concentrations (1 and 2 μM), the response shifted to apoptosis, which was consistent with the effect of AM6-36 on other apoptotic signatures including an increase of apoptotic annexin V(+) 7-AAD(-) cells, loss of mitochondrial membrane potential, induction of poly(ADP-ribose) polymerase cleavage, and activation of several caspases. These apoptotic effects are potentially due to up-regulation of p38 MAPK and JNK phosphorylation and down-regulation of c-Myc oncogene expression. Taken together, AM6-36 might serve as an effective anticancer agent by inducing G2/M cell cycle arrest and apoptosis through the activation of MAPKs and inhibition of c-Myc.
Figures
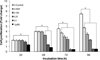


Similar articles
-
Induction of retinoid X receptor activity and consequent upregulation of p21WAF1/CIP1 by indenoisoquinolines in MCF7 cells.Cancer Prev Res (Phila). 2011 Apr;4(4):592-607. doi: 10.1158/1940-6207.CAPR-10-0004. Cancer Prev Res (Phila). 2011. PMID: 21464033 Free PMC article.
-
Novel indoloquinoline derivative, IQDMA, induces G(2)/M phase arrest and apoptosis in A549 cells through JNK/p38 MAPK signaling activation.Life Sci. 2009 Sep 23;85(13-14):505-16. doi: 10.1016/j.lfs.2009.08.006. Epub 2009 Aug 21. Life Sci. 2009. PMID: 19699753
-
Identification, synthesis, and biological evaluation of the metabolites of 3-amino-6-(3'-aminopropyl)-5H-indeno[1,2-c]isoquinoline-5,11-(6H)dione (AM6-36), a promising rexinoid lead compound for the development of cancer chemotherapeutic and chemopreventive agents.J Med Chem. 2012 Jun 28;55(12):5965-81. doi: 10.1021/jm3006806. Epub 2012 Jun 19. J Med Chem. 2012. PMID: 22712432 Free PMC article.
-
3,6-Dihydroxyflavone induces apoptosis in leukemia HL-60 cell via reactive oxygen species-mediated p38 MAPK/JNK pathway.Eur J Pharmacol. 2010 Dec 1;648(1-3):31-8. doi: 10.1016/j.ejphar.2010.08.020. Epub 2010 Sep 16. Eur J Pharmacol. 2010. PMID: 20840847
-
A link between benzyl isothiocyanate-induced cell cycle arrest and apoptosis: involvement of mitogen-activated protein kinases in the Bcl-2 phosphorylation.Cancer Res. 2004 Mar 15;64(6):2134-42. doi: 10.1158/0008-5472.can-03-2296. Cancer Res. 2004. PMID: 15026354
Cited by
-
A review of the molecular design and biological activities of RXR agonists.Med Res Rev. 2019 Jul;39(4):1372-1397. doi: 10.1002/med.21578. Epub 2019 Apr 3. Med Res Rev. 2019. PMID: 30941786 Free PMC article. Review.
-
Skeletal muscle PGC-1α controls whole-body lactate homeostasis through estrogen-related receptor α-dependent activation of LDH B and repression of LDH A.Proc Natl Acad Sci U S A. 2013 May 21;110(21):8738-43. doi: 10.1073/pnas.1212976110. Epub 2013 May 6. Proc Natl Acad Sci U S A. 2013. PMID: 23650363 Free PMC article.
-
(E)-2,4-bis(p-hydroxyphenyl)-2-butenal has an antiproliferative effect on NSCLC cells induced by p38 MAPK-mediated suppression of NF-κB and up-regulation of TNFRSF10B (DR5).Br J Pharmacol. 2013 Mar;168(6):1471-84. doi: 10.1111/bph.12024. Br J Pharmacol. 2013. PMID: 23082969 Free PMC article.
-
Ellipticine derivative induces potent cytostatic effect in acute myeloid leukaemia cells.Invest New Drugs. 2014 Dec;32(6):1113-22. doi: 10.1007/s10637-014-0140-3. Epub 2014 Aug 10. Invest New Drugs. 2014. PMID: 25107543
-
Research on the effect of ginseng polysaccharide on apoptosis and cell cycle of human leukemia cell line K562 and its molecular mechanisms.Exp Ther Med. 2017 Mar;13(3):924-934. doi: 10.3892/etm.2017.4087. Epub 2017 Jan 24. Exp Ther Med. 2017. PMID: 28450921 Free PMC article.
References
-
- Giri P, Kumar GS. Mini Rev. Med. Chem. 2010;10:568–577. - PubMed
-
- Hsu WH, Hsieh YS, Kuo HC, Teng CY, Huang HI, Wang CJ, Yang SF, Liou YS, Kuo WH. Arch. Toxicol. 2007;81:719–728. - PubMed
-
- Malikova J, Zdarilova A, Hlobilkova A. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 2006;150:5–12. - PubMed
-
- Konkimalla VB, Efferth T. Biochem. Pharmacol. 2010;79:1092–1099. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

