Parallel β-sheet secondary structure is stabilized and terminated by interstrand disulfide cross-linking
- PMID: 22148521
- PMCID: PMC3266109
- DOI: 10.1021/ja208856c
Parallel β-sheet secondary structure is stabilized and terminated by interstrand disulfide cross-linking
Abstract
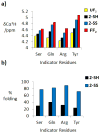
Disulfide bonds between Cys residues in adjacent strands of parallel β-sheets are rare among proteins, which suggests that parallel β-sheet structure is not stabilized by such disulfide cross-links. We report experimental results that show, surprisingly, that an interstrand disulfide bond can stabilize parallel β-sheets formed by an autonomously folding peptide in aqueous solution. NMR analysis reveals that parallel β-sheet structure is terminated beyond the disulfide bond, which causes deviation from the extended backbone conformation at one of the Cys residues.
© 2011 American Chemical Society
Figures

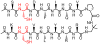



Similar articles
-
Context-dependence of the contribution of disulfide bonds to beta-hairpin stability.Chemistry. 2008;14(2):488-99. doi: 10.1002/chem.200700845. Chemistry. 2008. PMID: 17943702
-
Disulfide-Mediated β-Strand Dimers: Hyperstable β-Sheets Lacking Tertiary Interactions and Turns.J Am Chem Soc. 2015 Apr 29;137(16):5363-71. doi: 10.1021/ja5117809. Epub 2015 Apr 16. J Am Chem Soc. 2015. PMID: 25835058 Free PMC article.
-
Design and construction of an open multistranded beta-sheet polypeptide stabilized by a disulfide bridge.J Am Chem Soc. 2002 May 8;124(18):4987-94. doi: 10.1021/ja0174276. J Am Chem Soc. 2002. PMID: 11982362
-
Design of monomeric water-soluble β-hairpin and β-sheet peptides.Methods Mol Biol. 2014;1216:15-52. doi: 10.1007/978-1-4939-1486-9_2. Methods Mol Biol. 2014. PMID: 25213409 Review.
-
Folding of peptides and proteins: role of disulfide bonds, recent developments.Biomol Concepts. 2013 Dec;4(6):597-604. doi: 10.1515/bmc-2013-0022. Biomol Concepts. 2013. PMID: 25436759 Review.
Cited by
-
Tightening up the structure, lighting up the pathway: Application of molecular constraints and light to manipulate protein folding, self-assembly and function.Sci China Chem. 2014 Dec;57(12):1615-1624. doi: 10.1007/s11426-014-5225-5. Sci China Chem. 2014. PMID: 25722715 Free PMC article.
-
Effect of Glycosylation on an Immunodominant Region in the V1V2 Variable Domain of the HIV-1 Envelope gp120 Protein.PLoS Comput Biol. 2016 Oct 7;12(10):e1005094. doi: 10.1371/journal.pcbi.1005094. eCollection 2016 Oct. PLoS Comput Biol. 2016. PMID: 27716795 Free PMC article.
-
Bicyclic β-Sheet Mimetics that Target the Transcriptional Coactivator β-Catenin and Inhibit Wnt Signaling.Angew Chem Int Ed Engl. 2021 Jun 14;60(25):13937-13944. doi: 10.1002/anie.202102082. Epub 2021 May 5. Angew Chem Int Ed Engl. 2021. PMID: 33783110 Free PMC article.
-
Macrocyclic β-Sheets Stabilized by Hydrogen Bond Surrogates.Angew Chem Int Ed Engl. 2023 Oct 9;62(41):e202303943. doi: 10.1002/anie.202303943. Epub 2023 Jun 2. Angew Chem Int Ed Engl. 2023. PMID: 37170337 Free PMC article.
-
Structure-Property Relationship Study of N-(Hydroxy)Peptides for the Design of Self-Assembled Parallel β-Sheets.J Org Chem. 2020 Oct 2;85(19):12329-12342. doi: 10.1021/acs.joc.0c01441. Epub 2020 Sep 17. J Org Chem. 2020. PMID: 32881524 Free PMC article.
References
-
- Henchey LK, Jochim AL, Arora PS. J Am Chem Soc. 2008;12:692–697. - PMC - PubMed
- Phelan JC, Skelton NJ, Braisted AC, McDowell RS. J Am Chem Soc. 1997;119:455–460.
- Haubner R, Schmitt W, Holzemann G, Goodman SL, Jonczyk A, Kessler H. J Am Chem Soc. 1996;118:7881–7891.
- Lutgring R, Chmielewski J. J Am Chem Soc. 1994;116:6451–6452.
- Betz SF. Protein Sci. 1993;2:1551–1558. - PMC - PubMed
- Harrison PM, Sternberg MJE. J Mol Biol. 1994;244:448–463. - PubMed
-
-
Selected examples: Felix AM, Heimer EP, Wang CT, Lambros TJ, Fournier A, Mowles TF, Maines S, Campbell RM, Wegrzynski BB, Toome V, Fry D, Madison VS. Int J Pept Protein Res. 1988;32:441–454.Osapay G, Taylor JW. J Am Chem Soc. 1992;114:6966–6973.Leduc AM, Trent JO, Wittlif JL, Bramlett KS, Briggs SL, Chirgadze NY, Wang Y, Burris TP, Spatola AF. Proc Natl Acad Sci USA. 2003;100:11273–11278.Zhou HXX, Hull LA, Kallenbach NR, Mayne L, Bai YW, Englander SW. J Am Chem Soc. 1994;116:6482–6483.Jackson D, King D, Chmielewski J, Singh S, Schultz P. J Am Chem Soc. 1991;113:9391–9392.
-
-
- McDonald NQ, Hendrickson WA. Cell. 1993:421–424. - PubMed
- Willey JM, van der Donk WA. Annu Rev Microbiol. 2007;61:477–501. - PubMed
- Ganz T. Nat Rev Immun. 2003;3:710–720. - PubMed
- Cheek S, Krishna SS, Grishin NV. J Mol Biol. 2006;359:215–237. - PubMed
- Lin SL, Nussinov R. Nature Struct Biol. 1995;2:835–837. - PubMed
- Gause GF, Brazhnikova MG. Nature. 1944;154:703.
-
-
Selected examples: Ghadiri MR, Choi C. J Am Chem Soc. 1990;112:1630–1632.Ruan FQ, Chen YQ, Hopkins PB. J Am Chem Soc. 1990;112:9403–9404.Tatko CD, Waters ML. J Am Chem Soc. 2002;124:9372–9373.Kelso MJ, Beyer RL, Hoangt HN, Lakdawala AS, Snyder JP, Oliver WV, Robertson TA, Appleton TG, Fairlie DP. J Am Chem Soc. 2004;126:4828–4842.Blackwell HE, Grubbs RH. Angew Chem Int Ed Engl. 1998;37:3281–3284.Bernal F, Tyler AF, Korsmeyer SJ, Walensky LD, Verdine GL. J Am Chem Soc. 2007;129:2456–2457.Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Science. 2004;305:1466–1470.Nowick JS. Acc Chem Res. 2008;41:1319–1330.Fujimoto K, Kajino M, Inouye M. Chem Eur J. 2008;14:857–863.Liu J, Wang D, Zheng Q, Lu M, Arora PS. J Am Chem Soc. 2008;130:4334–4337.Wang D, Chen K, Kulp JL, III, Arora PS. J Am Chem Soc. 2006;128:9248–9256.Haney CM, Loch MT, Horne WS. Chem Commun. 2011 doi: 10.1039/C1CC12010G.Davies JS. J Pept Sci. 2003;9:471–501.Miller SJ, Blackwell HE, Grubbs RH. J Am Chem Soc. 1996;118:9606–9614.Schafmeister CE, Po J, Verdine GL. J Am Chem Soc. 2000;122:5891–5892.Brunel FM, Dawson PE. Chem Commun. 2005:2552–2554.Judice JK, Tom JY, Huang W, Wrin T, Vennari J, Petropoulos CJ, McDowell RS. Proc Natl Acad Sci USA. 1997;94:13426–13430.Sia SK, Carr PA, Cochran AG, Malashkevich VN, Kim PS. Proc Natl Acad Sci USA. 2002;99:14664–14669.Balaram H, Uma K, Balaram P. Int J Peptide Protein Res. 1990;35:495–500.Holland-Nell K, Meldal M. Angew Chem. 2011;123:5310–5312.Liu C, Sawaya MR, Cheng PN, Zheng J, Nowick JS, Eisenberg D. J Am Chem Soc. 2011;133:6736–6744.
-
-
- Hutchinson EG, Sessions RB, Thornton JM, Woolfson DN. Protein Sci. 1998;7:2287–2300. - PMC - PubMed
- Wouters MA, Curmi PMG. Proteins: Struct Funct Genet. 22:119–131. - PubMed
- Lifson S, Sander C. J Mol Biol. 1980;139:627–639. - PubMed
- Richardson JS. Advanc Protein Chem. 1981;34:167–339. - PubMed
- Harrison PM, Sternberg MJ. J Mol Biol. 1996;264:603–623. - PubMed
- Srinivisan N, Sowdhamini R, Ramakrishnan C, Balaram P. Int J Pept Protein Res. 1990;36:147–155. - PubMed
- Mao B. J Am Chem Soc. 1988;111:6132–6136.
- Chou PY, Fasman GD. Biochemistry. 1974;13:221–222. - PubMed
- Levitt M. Biochemistry. 1978;17:4277–4285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

