Cyclostreptin derivatives specifically target cellular tubulin and further map the paclitaxel site
- PMID: 22148836
- PMCID: PMC3255483
- DOI: 10.1021/bi201380p
Cyclostreptin derivatives specifically target cellular tubulin and further map the paclitaxel site
Abstract
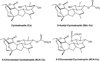
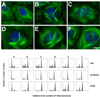
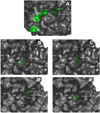
Cyclostreptin is the first microtubule-stabilizing agent whose mechanism of action was discovered to involve formation of a covalent bond with tubulin. Treatment of cells with cyclostreptin irreversibly stabilizes their microtubules because cyclostreptin forms a covalent bond to β-tubulin at either the T220 or the N228 residue, located at the microtubule pore or luminal taxoid binding site, respectively. Because of its unique mechanism of action, cyclostreptin overcomes P-glycoprotein-mediated multidrug resistance in tumor cells. We used a series of reactive cyclostreptin analogues, 6-chloroacetyl-cyclostreptin, 8-chloroacetyl-cyclostreptin, and [(14)C-acetyl]-8-acetyl-cyclostreptin, to characterize the cellular target of the compound and to map the binding site. The three analogues were cytotoxic and stabilized microtubules in both sensitive and multidrug resistant tumor cells. In both types of cells, we identified β-tubulin as the only or the predominantly labeled cellular protein, indicating that covalent binding to microtubules is sufficient to prevent drug efflux mediated by P-glycoprotein. 6-Chloroacetyl-cyclostreptin, 8-chloroacetyl-cyclostreptin, and 8-acetyl-cyclostreptin labeled both microtubules and unassembled tubulin at a single residue of the same tryptic peptide of β-tubulin as was labeled by cyclostreptin (219-LTTPTYGDLNHLVSATMSGVTTCLR-243), but labeling with the analogues occurred at different positions of the peptide. 8-Acetyl-cyclostreptin reacted with either T220 or N228, as did the natural product, while 8-chloroacetyl-cyclostreptin formed a cross-link to C241. Finally, 6-chloroacetyl-cyclostreptin reacted with any of the three residues, thus labeling the pathway for cyclostreptin-like compounds, leading from the pore where these compounds enter the microtubule to the luminal binding pocket.
Figures




References
-
- Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. - PubMed
-
- Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–667. - PubMed
-
- Galsky MD, Dritselis A, Kirkpatrick P, Oh WK. Cabazitaxel. Nat Rev Drug Discov. 2010;9:677–678. - PubMed
-
- Dumontet C, Jordan MA, Lee FF. Ixabepilone: targeting βIII-tubulin expression in taxane-resistant malignancies. Mol Cancer Ther. 2009;8:17–25. - PubMed
-
- Pryor DE, O'Brate A, Bilcer G, Díaz JF, Wang Y, Kabaki M, Jung MK, Andreu JM, Ghosh AK, Giannakakou P, Hamel E. The microtubule stabilizing agent laulimalide does not bind in the taxoid site, kills cells resistant to paclitaxel and epothilones, and may not require its epoxide moiety for activity. Biochemistry. 2002;41:9109–9115. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

