Rhodium catalyzed chelation-assisted C-H bond functionalization reactions
- PMID: 22148885
- PMCID: PMC3307943
- DOI: 10.1021/ar200190g
Rhodium catalyzed chelation-assisted C-H bond functionalization reactions
Abstract
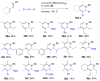
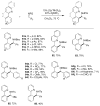
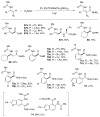
Over the last several decades, researchers have achieved remarkable progress in the field of organometallic chemistry. The development of metal-catalyzed cross-coupling reactions represents a paradigm shift in chemical synthesis, and today synthetic chemists can readily access carbon-carbon and carbon-heteroatom bonds from a vast array of starting compounds. Although we cannot understate the importance of these methods, the required prefunctionalization to carry out these reactions adds cost and reduces the availability of the starting reagents. The use of C-H bond activation in lieu of prefunctionalization has presented a tantalizing alternative to classical cross-coupling reactions. Researchers have met the challenges of selectivity and reactivity associated with the development of C-H bond functionalization reactions with an explosion of creative advances in substrate and catalyst design. Literature reports on selectivity based on steric effects, acidity, and electronic and directing group effects are now numerous. Our group has developed an array of C-H bond functionalization reactions that take advantage of a chelating directing group, and this Account surveys our progress in this area. The use of chelation control in C-H bond functionalization offers several advantages with respect to substrate scope and application to total synthesis. The predictability and decreased dependence on the inherent stereoelectronics of the substrate generally result in selective and high yielding transformations with broad applicability. The nature of the chelating moiety can be chosen to serve as a functional handle in subsequent elaborations. Our work began with the use of Rh(I) catalysts in intramolecular aromatic C-H annulations, which we further developed to include enantioselective transformations. The application of this chemistry to the simple olefinic C-H bonds found in α,β-unsaturated imines allowed access to highly substituted olefins, pyridines, and piperidines. We observed complementary reactivity with Rh(III) catalysts and developed an oxidative coupling with unactivated alkenes. Further studies on the Rh(III) catalysts led us to develop methods for the coupling of C-H bonds to polarized π bonds such as those in imines and isocyanates. In several cases the methods that we have developed for chelation-controlled C-H bond functionalization have been applied to the total synthesis of complex molecules such as natural products, highlighting the utility of these methods in organic synthesis.
Figures








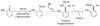



Similar articles
-
Electronic and Steric Tuning of a Prototypical Piano Stool Complex: Rh(III) Catalysis for C-H Functionalization.Acc Chem Res. 2018 Jan 16;51(1):170-180. doi: 10.1021/acs.accounts.7b00444. Epub 2017 Dec 22. Acc Chem Res. 2018. PMID: 29272106 Free PMC article. Review.
-
Transition-Metal-Catalyzed C-C Bond Formation from C-C Activation.Acc Chem Res. 2023 Nov 7;56(21):2867-2886. doi: 10.1021/acs.accounts.3c00230. Epub 2023 Oct 26. Acc Chem Res. 2023. PMID: 37882453
-
Mechanism of Rhodium-Catalyzed C-H Functionalization: Advances in Theoretical Investigation.Acc Chem Res. 2017 Nov 21;50(11):2799-2808. doi: 10.1021/acs.accounts.7b00400. Epub 2017 Nov 7. Acc Chem Res. 2017. PMID: 29112396
-
Substrate activation strategies in rhodium(III)-catalyzed selective functionalization of arenes.Acc Chem Res. 2015 Apr 21;48(4):1007-20. doi: 10.1021/acs.accounts.5b00077. Epub 2015 Apr 6. Acc Chem Res. 2015. PMID: 25844661
-
Direct functionalization of nitrogen heterocycles via Rh-catalyzed C-H bond activation.Acc Chem Res. 2008 Aug;41(8):1013-25. doi: 10.1021/ar800042p. Epub 2008 Jul 11. Acc Chem Res. 2008. PMID: 18616300 Free PMC article. Review.
Cited by
-
Rh(iii)-catalyzed tandem annulative redox-neutral arylation/amidation of aromatic tethered alkenes.Chem Sci. 2020 Oct 16;11(44):12124-12129. doi: 10.1039/d0sc04007j. Chem Sci. 2020. PMID: 34094427 Free PMC article.
-
Ligand-promoted alkylation of C(sp3)-H and C(sp2)-H bonds.J Am Chem Soc. 2014 Sep 24;136(38):13194-7. doi: 10.1021/ja508165a. Epub 2014 Sep 15. J Am Chem Soc. 2014. PMID: 25208210 Free PMC article.
-
An osmium(II) methane complex: Elucidation of the methane coordination mode.Sci Adv. 2023 Jun 9;9(23):eadg8130. doi: 10.1126/sciadv.adg8130. Epub 2023 Jun 9. Sci Adv. 2023. PMID: 37294762 Free PMC article.
-
Convergent Synthesis of Diverse Nitrogen Heterocycles via Rh(III)-Catalyzed C-H Conjugate Addition/Cyclization Reactions.Org Lett. 2016 Jul 1;18(13):3294-7. doi: 10.1021/acs.orglett.6b01611. Epub 2016 Jun 23. Org Lett. 2016. PMID: 27337641 Free PMC article.
-
Rh(III)-Catalyzed Cyclopropanation of Unactivated Olefins Initiated by C-H Activation.Synlett. 2019 Sep;30(15):1787-1790. doi: 10.1055/s-0039-1690130. Epub 2019 Jul 22. Synlett. 2019. PMID: 32801480 Free PMC article.
References
-
-
We recently published an Accounts review on the separate topic of direct functionalization of nitrogen heterocycles by Rh-catalyzed C-H bond functionalization: Lewis JC, Bergman RG, Ellman JA. Direct Functionalization of Nitrogen Heterocycles via Rh-Catalyzed C-H Bond Activation. Acc Chem Res. 2008;41:1013–1025.
-
-
-
For reviews on C-H bond functionalization, see the following and leading references therein: Crabtree RH. Introduction to Selective Functionalization of C-H Bonds. Chem Rev. 2010;110:575.Colby DA, Bergman RG, Ellman JA. Rhodium-Catalyzed C-C Bond Formation via Heteroatom-Directed C-H Bond Activation. Chem Rev. 2010;110:624–655.Bellina F, Rossi R. Transition Metal-Catalyzed Direct Arylation of Substrates with Activated sp3-Hybridized C-H Bonds and Some of Their Synthetic Equivalents with Aryl Halides and Pseudohalides. Chem Rev. 2010;110:1082–1146.Lyons TW, Sanford MS. Palladium-Catalyzed Ligand-Directed C-H Functionalization Reactions. Chem Rev. 2010;110:1147–1169.Davies HML, Du Bois J, Yu JQ. C-H Functionalization in Organic Synthesis. Chem Soc Rev. 2011;40:1855–1856.
-
-
- Jun CH, Moon CW, Lee DY. Chelation-Assisted Carbon–Hydrogen and Carbon–Carbon Bond Activation by Transition Metal Catalysts. Chem Eur J. 2002;8:2422–2428. - PubMed
- Kakiuchi F, Ohtaki H, Sonoda M, Chatani N, Murai S. Mechanistic Study of the Ru(H)2(CO)(PPh3)3-Catalyzed Addition of C-H Bonds in Aromatic Esters to Olefins. Chem Lett. 2001;30:918–919.
- Lenges CP, Brookhart M. Addition of Olefins to Aromatic Ketones Catalyzed by Rh(I) Olefin Complexes. J Am Chem Soc. 1999;121:6616–6623. - PubMed
- Matsubara T, Koga N, Musaev D, Morokuma K. Density Functional Study on Highly ortho-Selective Addition of an Aromatic C-H Bond to Olefins Catalyzed by a Ru(H)2 (CO)(PR3)3 Complex. Organometallics. 2000;19:2318–2329.
-
- Murai S, Kakiuchi F, Sekine S, Tanaka Y, Kamatani A, Sonoda M, Chatani N. Efficient Catalytic Addition of Aromatic Carbon-Hydrogen Bonds to Olefins. Nature. 1993;366:529–531.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

