Functionally diverse nucleophilic trapping of iminium intermediates generated utilizing visible light
- PMID: 22148974
- PMCID: PMC3253246
- DOI: 10.1021/ol202883v
Functionally diverse nucleophilic trapping of iminium intermediates generated utilizing visible light
Abstract
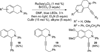
Our previous studies into visible-light-mediated aza-Henry reactions demonstrated that molecular oxygen played a vital role in catalyst turnover as well as the production of base to facilitate the nucleophilic addition of nitroalkanes. Herein, improved conditions for the generation of iminium ions from tetrahydroisoquinolines that allow for versatile nucleophilic trapping are reported. The new conditions provide access to a diverse range of functionality under mild, anaerobic reaction conditions as well as mechanistic insights into the photoredox cycle.
© 2011 American Chemical Society
Figures
References
-
-
For reviews on cross dehydrogenative coupling, see: Yeung CS, Dong VM. Chem. Rev. 2011;111:1215. Li C-J. Acc. Chem. Res. 2010;43:581. Li C-J. Acc. Chem. Res. 2009;42:335. Murahashi S-I, Zhang D. Chem. Soc. Rev. 2008;37:1490.
-
-
- Soriano MDPC, Shankaraiah N, Santos LS. Tetrahedron Lett. 2010;51:1770.
- Mahmoudian LS, Rahimi-Moghaddam P. Recent Patents on Anti-Cancer Drug Discovery. 2009;4:92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources