Serum proteins prevent aggregation of Fe2O3 and ZnO nanoparticles
- PMID: 22149273
- PMCID: PMC3963816
- DOI: 10.3109/17435390.2011.625131
Serum proteins prevent aggregation of Fe2O3 and ZnO nanoparticles
Abstract
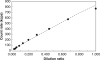
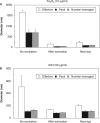
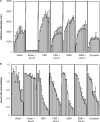
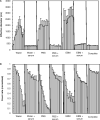
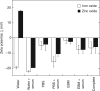
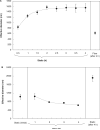
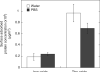
Aggregation of metal oxide nanoparticles in aqueous media complicates interpretation of in vitro studies of nanoparticle-cell interactions. We used dynamic light scattering to investigate the aggregation dynamics of iron oxide and zinc oxide nanoparticles. Our results show that iron oxide particles aggregate more readily than zinc oxide particles. Pretreatment with serum stabilises iron oxide and zinc oxide nanoparticles against aggregation. Serum-treated iron oxide is stable only in pure water, while zinc oxide is stable in water or cell culture media. These findings, combined with zeta potential measurements and quantification of proteins adsorbed on particle surface, suggest that serum stabilisation of iron oxide particles occurs primarily through protein adsorption and resulting net surface charge. Zinc oxide stabilisation, however, also involves steric hindrance of particle aggregation. Fluid shear at levels used in flow experiments breaks up iron oxide particle aggregates. These results enhance our understanding of nanoparticle aggregation and its consequences for research on the biological effects of nanomaterials.
Figures

Similar articles
-
Inulin as a novel biocompatible coating: evaluation of surface affinities toward CaHPO4, α-Fe2O3, ZnO, CaHPO4@ZnO and α-Fe2O3@ZnO nanoparticles.J Colloid Interface Sci. 2015 Dec 15;460:339-48. doi: 10.1016/j.jcis.2015.08.057. Epub 2015 Aug 24. J Colloid Interface Sci. 2015. PMID: 26364076
-
Characterisation of Fe-oxide nanoparticles coated with humic acid and Suwannee River natural organic matter.Sci Total Environ. 2013 Sep 1;461-462:19-27. doi: 10.1016/j.scitotenv.2013.04.083. Epub 2013 May 25. Sci Total Environ. 2013. PMID: 23712112
-
The effects of aggregation and protein corona on the cellular internalization of iron oxide nanoparticles.Biomaterials. 2011 Dec;32(35):9353-63. doi: 10.1016/j.biomaterials.2011.08.048. Epub 2011 Sep 10. Biomaterials. 2011. PMID: 21911254
-
Chemical design of biocompatible iron oxide nanoparticles for medical applications.Small. 2013 May 27;9(9-10):1450-66. doi: 10.1002/smll.201202111. Epub 2012 Dec 11. Small. 2013. PMID: 23233377 Review.
-
Potential Effects of Metal Oxides on Agricultural Production of Rice: A Mini Review.Plants (Basel). 2023 Feb 9;12(4):778. doi: 10.3390/plants12040778. Plants (Basel). 2023. PMID: 36840126 Free PMC article. Review.
Cited by
-
Iron oxide nanoparticle agglomeration influences dose rates and modulates oxidative stress-mediated dose-response profiles in vitro.Nanotoxicology. 2014 Sep;8(6):663-75. doi: 10.3109/17435390.2013.822115. Epub 2013 Jul 31. Nanotoxicology. 2014. PMID: 23837572 Free PMC article.
-
Comparison of Calcium Phosphate and Zinc Oxide Nanoparticles as Dermal Penetration Enhancers for Albumin.Indian J Pharm Sci. 2015 Nov-Dec;77(6):694-704. doi: 10.4103/0250-474x.174989. Indian J Pharm Sci. 2015. Retraction in: Indian J Pharm Sci. 2015 Nov-Dec;77(6):694. PMID: 26997697 Free PMC article. Retracted.
-
Zinc oxide nanoparticles induce necrosis and apoptosis in macrophages in a p47phox- and Nrf2-independent manner.PLoS One. 2013 Jun 3;8(6):e65704. doi: 10.1371/journal.pone.0065704. Print 2013. PLoS One. 2013. PMID: 23755271 Free PMC article.
-
Nanoparticle-nanoparticle interactions in biological media by atomic force microscopy.Langmuir. 2013 Sep 10;29(36):11385-95. doi: 10.1021/la4019585. Epub 2013 Aug 26. Langmuir. 2013. PMID: 23978039 Free PMC article.
-
Chemical screens for particle-induced macrophage death identifies kinase inhibitors of phagocytosis as targets for toxicity.J Nanobiotechnology. 2024 Oct 14;22(1):621. doi: 10.1186/s12951-024-02885-8. J Nanobiotechnology. 2024. PMID: 39396993 Free PMC article.
References
-
- Allouni ZE, Cimpan MR, Høl PJ, Skodvin T, Gjerdet NR. Agglomeration and sedimentation of TiO2 nanoparticles in cell culture medium. Coll Surf B. 2009;68:83–87. - PubMed
-
- Berg JM, Romoser A, Banerjee N, Zebda R, Sayes CM. The relationship between pH and zeta potential of 30 nm metal oxide nanoparticle suspensions relevant to in vitro toxicological evaluations. Nanotoxicology. 2009;3:276–283.
-
- Bergman L, Rosenholm J, Öst A-B, Duchanoy A, Kankaanpää P, Heino J, Lindén M. On the complexity of electrostatic suspension stabilization of functionalized silica nanoparticles for biotargeting and imaging applications. J Nanomaterials. 2008:712514.
-
- Deguchi S, Yamazaki T, Mukai S, Usami R, Horikoshi K. Stabilization of C60 nanoparticles by protein adsorption and its implications for toxicity studies. Chem Res Toxicol. 2007;20:854–858. - PubMed
-
- Deng ZJ, Mortimer G, Schiller T, Musumeci A, Martin D, Minchin RF. Differential plasma protein binding to metal oxide nanoparticles. Nanotechnology. 2009;20:455101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
