Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer
- PMID: 22149876
- PMCID: PMC5705195
- DOI: 10.1056/NEJMoa1109653
Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer
Abstract
Background: Resistance to endocrine therapy in breast cancer is associated with activation of the mammalian target of rapamycin (mTOR) intracellular signaling pathway. In early studies, the mTOR inhibitor everolimus added to endocrine therapy showed antitumor activity.
Methods: In this phase 3, randomized trial, we compared everolimus and exemestane versus exemestane and placebo (randomly assigned in a 2:1 ratio) in 724 patients with hormone-receptor-positive advanced breast cancer who had recurrence or progression while receiving previous therapy with a nonsteroidal aromatase inhibitor in the adjuvant setting or to treat advanced disease (or both). The primary end point was progression-free survival. Secondary end points included survival, response rate, and safety. A preplanned interim analysis was performed by an independent data and safety monitoring committee after 359 progression-free survival events were observed.
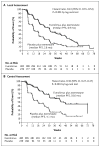
Results: Baseline characteristics were well balanced between the two study groups. The median age was 62 years, 56% had visceral involvement, and 84% had hormone-sensitive disease. Previous therapy included letrozole or anastrozole (100%), tamoxifen (48%), fulvestrant (16%), and chemotherapy (68%). The most common grade 3 or 4 adverse events were stomatitis (8% in the everolimus-plus-exemestane group vs. 1% in the placebo-plus-exemestane group), anemia (6% vs. <1%), dyspnea (4% vs. 1%), hyperglycemia (4% vs. <1%), fatigue (4% vs. 1%), and pneumonitis (3% vs. 0%). At the interim analysis, median progression-free survival was 6.9 months with everolimus plus exemestane and 2.8 months with placebo plus exemestane, according to assessments by local investigators (hazard ratio for progression or death, 0.43; 95% confidence interval [CI], 0.35 to 0.54; P<0.001). Median progression-free survival was 10.6 months and 4.1 months, respectively, according to central assessment (hazard ratio, 0.36; 95% CI, 0.27 to 0.47; P<0.001).
Conclusions: Everolimus combined with an aromatase inhibitor improved progression-free survival in patients with hormone-receptor-positive advanced breast cancer previously treated with nonsteroidal aromatase inhibitors. (Funded by Novartis; BOLERO-2 ClinicalTrials.gov number, NCT00863655.).
Figures

Comment in
-
Everolimus in HR-positive advanced breast cancer.N Engl J Med. 2012 May 3;366(18):1738-9; author reply 1739-40. doi: 10.1056/NEJMc1202719. N Engl J Med. 2012. PMID: 22551139 No abstract available.
-
Everolimus in HR-positive advanced breast cancer.N Engl J Med. 2012 May 3;366(18):1739; author reply 1739-40. doi: 10.1056/NEJMc1202719. N Engl J Med. 2012. PMID: 22551140 No abstract available.
References
-
- Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431–42. - PubMed
-
- Mouridsen H, Gershanovich M, Sun Y, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19:2596–606. [Erratum, J Clin Oncol 2001;19:3302.] - PubMed
-
- Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. J Clin Oncol. 2000;18:3758–67. - PubMed
-
- Bonneterre J, Thürlimann B, Robertson JF, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol. 2000;18:3748–57. - PubMed
-
- Mauri D, Pavlidis N, Polyzos NP, Ioannidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285–91. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
