Adenovirus-associated virus vector-mediated gene transfer in hemophilia B
- PMID: 22149959
- PMCID: PMC3265081
- DOI: 10.1056/NEJMoa1108046
Adenovirus-associated virus vector-mediated gene transfer in hemophilia B
Abstract
Background: Hemophilia B, an X-linked disorder, is ideally suited for gene therapy. We investigated the use of a new gene therapy in patients with the disorder.
Methods: We infused a single dose of a serotype-8-pseudotyped, self-complementary adenovirus-associated virus (AAV) vector expressing a codon-optimized human factor IX (FIX) transgene (scAAV2/8-LP1-hFIXco) in a peripheral vein in six patients with severe hemophilia B (FIX activity, <1% of normal values). Study participants were enrolled sequentially in one of three cohorts (given a high, intermediate, or low dose of vector), with two participants in each group. Vector was administered without immunosuppressive therapy, and participants were followed for 6 to 16 months.
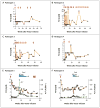
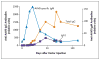
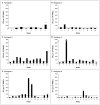
Results: AAV-mediated expression of FIX at 2 to 11% of normal levels was observed in all participants. Four of the six discontinued FIX prophylaxis and remained free of spontaneous hemorrhage; in the other two, the interval between prophylactic injections was increased. Of the two participants who received the high dose of vector, one had a transient, asymptomatic elevation of serum aminotransferase levels, which was associated with the detection of AAV8-capsid-specific T cells in the peripheral blood; the other had a slight increase in liver-enzyme levels, the cause of which was less clear. Each of these two participants received a short course of glucocorticoid therapy, which rapidly normalized aminotransferase levels and maintained FIX levels in the range of 3 to 11% of normal values.
Conclusions: Peripheral-vein infusion of scAAV2/8-LP1-hFIXco resulted in FIX transgene expression at levels sufficient to improve the bleeding phenotype, with few side effects. Although immune-mediated clearance of AAV-transduced hepatocytes remains a concern, this process may be controlled with a short course of glucocorticoids without loss of transgene expression. (Funded by the Medical Research Council and others; ClinicalTrials.gov number, NCT00979238.).
Figures
Comment in
-
Merry christmas for patients with hemophilia B.N Engl J Med. 2011 Dec 22;365(25):2424-5. doi: 10.1056/NEJMe1111138. Epub 2011 Dec 10. N Engl J Med. 2011. PMID: 22149960 No abstract available.
-
Ultrasound-targeted hepatic delivery of factor IX in hemophiliac mice.Gene Ther. 2016 Jun;23(6):510-9. doi: 10.1038/gt.2016.23. Epub 2016 Apr 7. Gene Ther. 2016. PMID: 26960037 Free PMC article.
References
-
- Nathwani AC, Tuddenham EG. Epidemiology of coagulation disorders. Baillieres Clin Haematol. 1992;5:383–439. - PubMed
-
- Darby SC, Kan SW, Spooner RJ, et al. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110:815–25. - PubMed
-
- Nathwani AC, Davidoff AM, Tuddenham EG. Prospects for gene therapy of haemophilia. Haemophilia. 2004;10:309–18. - PubMed
-
- Herzog RW, Yang EY, Couto LB, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. - PubMed
-
- Snyder RO, Miao C, Meuse L, et al. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med. 1999;5:64–70. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
