Inflammatory cortical demyelination in early multiple sclerosis
- PMID: 22150037
- PMCID: PMC3282172
- DOI: 10.1056/NEJMoa1100648
Inflammatory cortical demyelination in early multiple sclerosis
Abstract
Background: Cortical disease has emerged as a critical aspect of the pathogenesis of multiple sclerosis, being associated with disease progression and cognitive impairment. Most studies of cortical lesions have focused on autopsy findings in patients with long-standing, chronic, progressive multiple sclerosis, and the noninflammatory nature of these lesions has been emphasized. Magnetic resonance imaging studies indicate that cortical damage occurs early in the disease.
Methods: We evaluated the prevalence and character of demyelinating cortical lesions in patients with multiple sclerosis. Cortical tissues were obtained in passing during biopsy sampling of white-matter lesions. In most cases, biopsy was done with the use of stereotactic procedures to diagnose suspected tumors. Patients with sufficient cortex (138 of 563 patients screened) were evaluated for cortical demyelination. Using immunohistochemistry, we characterized cortical lesions with respect to demyelinating activity, inflammatory infiltrates, the presence of meningeal inflammation, and a topographic association between cortical demyelination and meningeal inflammation. Diagnoses were ascertained in a subgroup of 77 patients (56%) at the last follow-up visit (at a median of 3.5 years).
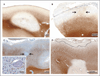
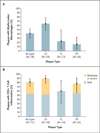
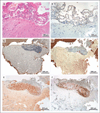
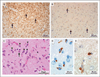
Results: Cortical demyelination was present in 53 patients (38%) (104 lesions and 222 tissue blocks) and was absent in 85 patients (121 tissue blocks). Twenty-five patients with cortical demyelination had definite multiple sclerosis (81% of 31 patients who underwent long-term follow-up), as did 33 patients without cortical demyelination (72% of 46 patients who underwent long-term follow-up). In representative tissues, 58 of 71 lesions (82%) showed CD3+ T-cell infiltrates, and 32 of 78 lesions (41%) showed macrophage-associated demyelination. Meningeal inflammation was topographically associated with cortical demyelination in patients who had sufficient meningeal tissue for study.
Conclusions: In this cohort of patients with early-stage multiple sclerosis, cortical demyelinating lesions were frequent, inflammatory, and strongly associated with meningeal inflammation. (Funded by the National Multiple Sclerosis Society and the National Institutes of Health.).
Figures

Comment in
-
Inflammation in multiple sclerosis--sorting out the gray matter.N Engl J Med. 2011 Dec 8;365(23):2231-3. doi: 10.1056/NEJMe1111945. N Engl J Med. 2011. PMID: 22150043 No abstract available.
-
Multiple sclerosis: 'Outside-in' demyelination in MS.Nat Rev Neurol. 2012 Jan 17;8(2):61. doi: 10.1038/nrneurol.2011.217. Nat Rev Neurol. 2012. PMID: 22249838 No abstract available.
References
-
- Lumsden C. The neuropathology of multiple sclerosis. In: Vinken P, Bruyn G, editors. Handbook of clinical neurology. New York: Elsevier; 1970. pp. 217–309.
-
- Kidd D, Barkhof F, McConnell R, Algra PR, Allen IV, Revesz T. Cortical lesions in multiple sclerosis. Brain. 1999;122:17–26. - PubMed
-
- Peterson JW, Bø L, Mørk S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400. - PubMed
-
- Bø L, Vedeler C, Nyland H, Trapp B, Mørk S. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler. 2003;9:323–331. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
