The molecular basis of herpes simplex virus latency
- PMID: 22150699
- PMCID: PMC3492847
- DOI: 10.1111/j.1574-6976.2011.00320.x
The molecular basis of herpes simplex virus latency
Abstract
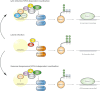
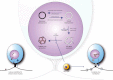
Herpes simplex virus type 1 is a neurotropic herpesvirus that establishes latency within sensory neurones. Following primary infection, the virus replicates productively within mucosal epithelial cells and enters sensory neurones via nerve termini. The virus is then transported to neuronal cell bodies where latency can be established. Periodically, the virus can reactivate to resume its normal lytic cycle gene expression programme and result in the generation of new virus progeny that are transported axonally back to the periphery. The ability to establish lifelong latency within the host and to periodically reactivate to facilitate dissemination is central to the survival strategy of this virus. Although incompletely understood, this review will focus on the mechanisms involved in the regulation of latency that centre on the functions of the virus-encoded latency-associated transcripts (LATs), epigenetic regulation of the latent virus genome and the molecular events that precipitate reactivation.
© 2011 Federation of European Microbiological Societies. Published by Blackwell Publishing Ltd. All rights reserved.
Figures

References
-
- Amelio AL, Giordani NV, Kubat NJ, O'neil JE, Bloom DC. Deacetylation of the herpes simplex virus type 1 latency-associated transcript (LAT) enhancer and a decrease in LAT abundance precede an increase in ICP0 transcriptional permissiveness at early times postexplant. J Virol. 2006;80:2063–2068. - PMC - PubMed
-
- Arthur J, Efstathiou S, Simmons A. Intranuclear foci containing low abundance herpes simplex virus latency-associated transcripts visualized by non-isotopic in situ hybridization. J Gen Virol. 1993;74(Pt 7):1363–1370. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

