The BB0646 protein demonstrates lipase and haemolytic activity associated with Borrelia burgdorferi, the aetiological agent of Lyme disease
- PMID: 22151008
- PMCID: PMC3256266
- DOI: 10.1111/j.1365-2958.2011.07932.x
The BB0646 protein demonstrates lipase and haemolytic activity associated with Borrelia burgdorferi, the aetiological agent of Lyme disease
Abstract
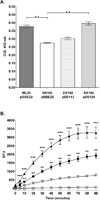
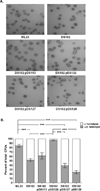
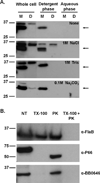
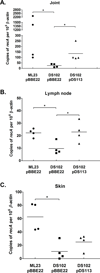
The etiological agent of Lyme disease, Borrelia burgdorferi, is transmitted by ticks of the Ixodes genus and, if untreated, can cause significant morbidity in affected individuals. Recent reports have shown that polyunsaturated fatty acids in the B. burgdorferi cell envelope are potential targets for oxidative damage, which can be lethal. How B. burgdorferi responds to this assault is not known. Herein we report evidence that bb0646 codes for a lipase that is located within the bosR operon and that has specificity for both saturated and polyunsaturated fatty acids. Specifically, strains harbouring mutated copies of the lipase, either in the form of an insertionally inactivated construct or site-directed mutations within the active site, demonstrated attenuated lipolytic and haemolytic phenotypes when compared with the isogenic parent and trans-complements. In vivo analysis showed that while the bb0646 mutant remains infectious, the spirochaetal load is significantly lower than both the isogenic parent and the complemented mutant strains. Taken together, these data demonstrate that BB0646 is a broad substrate specific lipase that contributes to lipolytic and haemolytic activity in vitro and is required for optimal B. burgdorferi infection.
© 2011 Blackwell Publishing Ltd.
Figures




Similar articles
-
BB0562 is a nutritional virulence determinant with lipase activity important for Borrelia burgdorferi infection and survival in fatty acid deficient environments.PLoS Pathog. 2021 Aug 20;17(8):e1009869. doi: 10.1371/journal.ppat.1009869. eCollection 2021 Aug. PLoS Pathog. 2021. PMID: 34415955 Free PMC article.
-
Influence of arthritis-related protein (BBF01) on infectivity of Borrelia burgdorferi B31.BMC Microbiol. 2013 May 7;13:100. doi: 10.1186/1471-2180-13-100. BMC Microbiol. 2013. PMID: 23651628 Free PMC article.
-
The Lon-1 Protease Is Required by Borrelia burgdorferi To Infect the Mammalian Host.Infect Immun. 2020 May 20;88(6):e00951-19. doi: 10.1128/IAI.00951-19. Print 2020 May 20. Infect Immun. 2020. PMID: 32205400 Free PMC article.
-
Transposon mutagenesis as an approach to improved understanding of Borrelia pathogenesis and biology.Front Cell Infect Microbiol. 2014 May 20;4:63. doi: 10.3389/fcimb.2014.00063. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24904839 Free PMC article. Review.
-
Borrelia burgdorferi protein interactions critical for microbial persistence in mammals.Cell Microbiol. 2019 Feb;21(2):e12885. doi: 10.1111/cmi.12885. Epub 2018 Jul 8. Cell Microbiol. 2019. PMID: 29934966 Free PMC article. Review.
Cited by
-
Identification of the minimal cytolytic unit for streptolysin S and an expansion of the toxin family.BMC Microbiol. 2015 Jul 24;15:141. doi: 10.1186/s12866-015-0464-y. BMC Microbiol. 2015. PMID: 26204951 Free PMC article.
-
Borrelia burgdorferi and tick proteins supporting pathogen persistence in the vector.Future Microbiol. 2013 Jan;8(1):41-56. doi: 10.2217/fmb.12.121. Future Microbiol. 2013. PMID: 23252492 Free PMC article. Review.
-
BB0562 is a nutritional virulence determinant with lipase activity important for Borrelia burgdorferi infection and survival in fatty acid deficient environments.PLoS Pathog. 2021 Aug 20;17(8):e1009869. doi: 10.1371/journal.ppat.1009869. eCollection 2021 Aug. PLoS Pathog. 2021. PMID: 34415955 Free PMC article.
-
In Vivo Imaging Demonstrates That Borrelia burgdorferi ospC Is Uniquely Expressed Temporally and Spatially throughout Experimental Infection.PLoS One. 2016 Sep 9;11(9):e0162501. doi: 10.1371/journal.pone.0162501. eCollection 2016. PLoS One. 2016. PMID: 27611840 Free PMC article.
-
Lyme Disease Pathogenesis.Curr Issues Mol Biol. 2021;42:473-518. doi: 10.21775/cimb.042.473. Epub 2020 Dec 23. Curr Issues Mol Biol. 2021. PMID: 33353871 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

