Projections and interconnections of genetically defined serotonin neurons in mice
- PMID: 22151329
- PMCID: PMC3268345
- DOI: 10.1111/j.1460-9568.2011.07936.x
Projections and interconnections of genetically defined serotonin neurons in mice
Abstract
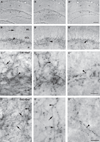
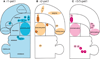
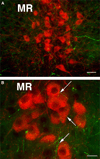
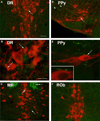
Brain serotonin neurons are heterogeneous and can be distinguished by several anatomical and physiological characteristics. Toward resolving this heterogeneity into classes of functional relevance, subtypes of mature serotonin neurons were previously identified based on gene expression differences initiated during development in different rhombomeric (r) segments of the hindbrain. This redefinition of mature serotonin neuron subtypes based on the criteria of genetic lineage, along with the enabling genetic fate mapping tools, now allows various functional properties, such as axonal projections, to be allocated onto these identified subtypes. Furthermore, our approach uniquely enables interconnections between the different serotonin neuron subtypes to be determined; this is especially relevant because serotonin neuron activity is regulated by several feedback mechanisms. We used intersectional and subtractive genetic fate mapping tools to generate three independent lines of mice in which serotonin neurons arising in different rhombomeric segments, either r1, r2 or both r3 and r5, were uniquely distinguished from all other serotonin neurons by their expression of enhanced green fluorescent protein. Each of these subgroups of serotonergic neurons had a unique combination of forebrain projection targets. Typically more than one subgroup innervated an individual target area. Unique patterns of interconnections between the different groups of serotonin neurons were also observed and these pathways could subserve feedback regulatory circuits. Overall, the current findings suggest that activation of subsets of serotonin neurons could result in topographic serotonin release in the forebrain coupled with feedback inhibition of serotonin neurons with alternative projection targets.
© 2011 The Authors. European Journal of Neuroscience © 2011 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Figures






Comment in
-
Dissecting the projections of different groups of raphe serotonergic neurons (commentary on Bang et al.).Eur J Neurosci. 2012 Jan;35(1):83-4. doi: 10.1111/j.1460-9568.2011.07983.x. Eur J Neurosci. 2012. PMID: 22211743 No abstract available.
References
-
- Awatramani R, Soriano P, Rodriguez C, Mai JJ, Dymecki SM. Cryptic boundaries in roof plate and choroid plexus identified by intersectional gene activation. Nat. Genet. 2003;35:70–75. - PubMed
-
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. - PubMed
-
- Bland ST, Hargrave D, Pepin JL, Amat J, Watkins LR, Maier SF. Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology. 2003;28:1589–1596. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

