Roles of quaternary structure and cysteine residues in the activity of human serine racemase
- PMID: 22151352
- PMCID: PMC3252284
- DOI: 10.1186/1471-2091-12-63
Roles of quaternary structure and cysteine residues in the activity of human serine racemase
Abstract
Background: D-serine is an important coagonist at the NR1 subunit of the NMDA receptor class of glutamate receptors. It is chiefly synthesized in the CNS by serine racemase (SR). Regulation of SR activity is still poorly understood. As step toward developing reagents and methods for investigating SR in vitro, we analyzed structure-function relationships of a recombinant enzyme of human sequence.
Results: Michaelis-Menten kinetic analysis indicated a KM value of 14 mM and Vmax value of 3.66 μmol·mg⁻¹·hr⁻¹ when L-serine was used as a substrate for purified SR. Gel-filtration chromatography and protein cross-linking experiments revealed that dimer is the major oligomeric form of recombinant SR in aqueous solution, though the proportions of monomer, tetramer, and larger aggregates differed somewhat with the specific buffer used. These buffers also altered activity in a manner correlating with the relative abundance of dimer. Activity assays showed that the dimeric gel-filtration fraction held the highest activity. Chemical reduction with DTT increased the activity of SR by elevating Vmax; cystamine, a reagent that blocks sulfhydryl groups, abolished SR activity. Gel-filtration chromatography and western blot analysis indicated that DTT enhanced the recovery of noncovalent SR dimer.
Conclusions: These data suggest that SR is most active as a noncovalent dimer containing one or more free sulfhydryls in the enzyme's active center or a modulatory site. Buffer composition and reduction/oxidation status during preparation can dramatically impact interpretations of SR activity. These findings also highlight the possibility that SR is sensitive to oxidative stress in vivo.
Figures
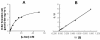

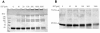







Similar articles
-
Cross-linking of serine racemase dimer by reactive oxygen species and reactive nitrogen species.J Neurosci Res. 2012 Jun;90(6):1218-29. doi: 10.1002/jnr.22832. Epub 2012 Feb 22. J Neurosci Res. 2012. PMID: 22354542 Free PMC article.
-
Direct calcium binding results in activation of brain serine racemase.J Biol Chem. 2002 Aug 2;277(31):27782-92. doi: 10.1074/jbc.M111814200. Epub 2002 May 20. J Biol Chem. 2002. PMID: 12021263
-
Recombinant human serine racemase: enzymologic characterization and comparison with its mouse ortholog.Protein Expr Purif. 2009 Jan;63(1):62-7. doi: 10.1016/j.pep.2008.09.003. Epub 2008 Sep 11. Protein Expr Purif. 2009. PMID: 18812225
-
D-amino acids in the brain: the biochemistry of brain serine racemase.FEBS J. 2008 Jul;275(14):3538-45. doi: 10.1111/j.1742-4658.2008.06517.x. FEBS J. 2008. PMID: 18564178 Review.
-
Serine racemase and the serine shuttle between neurons and astrocytes.Biochim Biophys Acta. 2011 Nov;1814(11):1558-66. doi: 10.1016/j.bbapap.2011.01.001. Epub 2011 Jan 9. Biochim Biophys Acta. 2011. PMID: 21224019 Review.
Cited by
-
Peripheral Biomarker for Vascular Disorders.Biomark Insights. 2018 Nov 29;13:1177271918812467. doi: 10.1177/1177271918812467. eCollection 2018. Biomark Insights. 2018. PMID: 30546256 Free PMC article.
-
One-step purification of twin-strep-tagged proteins and their complexes on strep-tactin resin cross-linked with bis(sulfosuccinimidyl) suberate (BS3).J Vis Exp. 2014 Apr 20;(86):51536. doi: 10.3791/51536. J Vis Exp. 2014. PMID: 24796313 Free PMC article.
-
D-Serine Production, Degradation, and Transport in ALS: Critical Role of Methodology.Neurol Res Int. 2012;2012:625245. doi: 10.1155/2012/625245. Epub 2012 Sep 16. Neurol Res Int. 2012. PMID: 23029613 Free PMC article.
-
The Energy Landscape of Human Serine Racemase.Front Mol Biosci. 2019 Jan 9;5:112. doi: 10.3389/fmolb.2018.00112. eCollection 2018. Front Mol Biosci. 2019. PMID: 30687716 Free PMC article. Review.
-
Cross-linking of serine racemase dimer by reactive oxygen species and reactive nitrogen species.J Neurosci Res. 2012 Jun;90(6):1218-29. doi: 10.1002/jnr.22832. Epub 2012 Feb 22. J Neurosci Res. 2012. PMID: 22354542 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

