Fatty acyl-CoA reductases of birds
- PMID: 22151413
- PMCID: PMC3265415
- DOI: 10.1186/1471-2091-12-64
Fatty acyl-CoA reductases of birds
Abstract
Background: Birds clean and lubricate their feathers with waxes that are produced in the uropygial gland, a holocrine gland located on their back above the tail. The type and the composition of the secreted wax esters are dependent on the bird species, for instance the wax ester secretion of goose contains branched-chain fatty acids and unbranched fatty alcohols, whereas that of barn owl contains fatty acids and alcohols both of which are branched. Alcohol-forming fatty acyl-CoA reductases (FAR) catalyze the reduction of activated acyl groups to fatty alcohols that can be esterified with acyl-CoA thioesters forming wax esters.
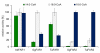
Results: cDNA sequences encoding fatty acyl-CoA reductases were cloned from the uropygial glands of barn owl (Tyto alba), domestic chicken (Gallus gallus domesticus) and domestic goose (Anser anser domesticus). Heterologous expression in Saccharomyces cerevisiae showed that they encode membrane associated enzymes which catalyze a NADPH dependent reduction of acyl-CoA thioesters to fatty alcohols. By feeding studies of transgenic yeast cultures and in vitro enzyme assays with membrane fractions of transgenic yeast cells two groups of isozymes with different properties were identified, termed FAR1 and FAR2. The FAR1 group mainly synthesized 1-hexadecanol and accepted substrates in the range between 14 and 18 carbon atoms, whereas the FAR2 group preferred stearoyl-CoA and accepted substrates between 16 and 20 carbon atoms. Expression studies with tissues of domestic chicken indicated that FAR transcripts were not restricted to the uropygial gland.
Conclusion: The data of our study suggest that the identified and characterized avian FAR isozymes, FAR1 and FAR2, can be involved in wax ester biosynthesis and in other pathways like ether lipid synthesis.
Figures





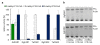

References
-
- Riendeau D, Rodriguez A, Meighen E. Resolution of the fatty acid reductase from Photobacterium phosphoreum into acyl protein synthetase and acyl-CoA reductase activities. Evidence for an enzyme complex. J Biol Chem. 1982;257(12):6908–6915. - PubMed
-
- Rodriguez A, Riendeau D, Meighen E. Purification of the acyl coenzyme A reductase component from a complex responsible for the reduction of fatty acids in biolumniscent bacteria. Properties and acyltransferase activity. J Biol Chem. 1983;258(8):5233–5237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

