Platelet-derived growth factor receptor-α cells in mouse urinary bladder: a new class of interstitial cells
- PMID: 22151424
- PMCID: PMC3822840
- DOI: 10.1111/j.1582-4934.2011.01506.x
Platelet-derived growth factor receptor-α cells in mouse urinary bladder: a new class of interstitial cells
Abstract
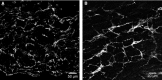
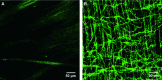
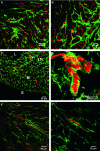
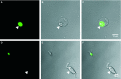
Specific classes of interstitial cells exist in visceral organs and have been implicated in several physiological functions including pacemaking and mediators in neurotransmission. In the bladder, Kit(+) interstitial cells have been reported to exist and have been suggested to be neuromodulators. More recently a second interstitial cell, which is identified using antibodies against platelet-derived growth factor receptor-α (PDGFR-α) has been described in the gastrointestinal (GI) tract and has been implicated in enteric motor neurotransmission. In this study, we examined the distribution of PDGFR-α(+) cells in the murine urinary bladder and the relation that these cells may have with nerve fibres and smooth muscle cells. Platelet-derived growth factor receptor-α(+) cells had a spindle shape or stellate morphology and often possessed multiple processes that contacted one another forming a loose network. These cells were distributed throughout the bladder wall, being present in the lamina propria as well as throughout the muscularis of the detrusor. These cells surrounded and were located between smooth muscle bundles and often came into close morphological association with intramural nerve fibres. These data describe a new class of interstitial cells that express a specific receptor within the bladder wall and provide morphological evidence for a possible neuromodulatory role in bladder function.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures




Similar articles
-
Morphological expression of KIT positive interstitial cells of Cajal in human bladder.J Urol. 2010 Jul;184(1):370-7. doi: 10.1016/j.juro.2010.03.005. Epub 2010 May 20. J Urol. 2010. PMID: 20488490 Free PMC article.
-
Properties of SK3 channel-expressing PDGFRα (+) cells in the rodent urinary bladder.Eur J Pharmacol. 2019 Oct 5;860:172552. doi: 10.1016/j.ejphar.2019.172552. Epub 2019 Jul 18. Eur J Pharmacol. 2019. PMID: 31326376
-
Identification of different phenotypes of interstitial cells in the upper and deep lamina propria of the human bladder dome.J Urol. 2014 Nov;192(5):1555-63. doi: 10.1016/j.juro.2014.05.096. Epub 2014 Jun 2. J Urol. 2014. PMID: 24893312
-
Interstitial cells in the urinary bladder--localization and function.Neurourol Urodyn. 2010;29(1):82-7. doi: 10.1002/nau.20739. Neurourol Urodyn. 2010. PMID: 20025023 Review.
-
The Mystery of the Interstitial Cells in the Urinary Bladder.Annu Rev Pharmacol Toxicol. 2018 Jan 6;58:603-623. doi: 10.1146/annurev-pharmtox-010617-052615. Epub 2017 Oct 6. Annu Rev Pharmacol Toxicol. 2018. PMID: 28992432 Review.
Cited by
-
Rhythmic Calcium Events in the Lamina Propria Network of the Urinary Bladder of Rat Pups.Front Syst Neurosci. 2017 Dec 11;11:87. doi: 10.3389/fnsys.2017.00087. eCollection 2017. Front Syst Neurosci. 2017. PMID: 29321730 Free PMC article.
-
A novel class of interstitial cells in the mouse and monkey female reproductive tracts.Biol Reprod. 2015 Apr;92(4):102. doi: 10.1095/biolreprod.114.124388. Epub 2015 Mar 18. Biol Reprod. 2015. PMID: 25788664 Free PMC article.
-
Studies of ultrastructure, gene expression, and marker analysis reveal that mouse bladder PDGFRA+ interstitial cells are fibroblasts.Am J Physiol Renal Physiol. 2022 Sep 1;323(3):F299-F321. doi: 10.1152/ajprenal.00135.2022. Epub 2022 Jul 14. Am J Physiol Renal Physiol. 2022. PMID: 35834272 Free PMC article.
-
The Urothelium: Life in a Liquid Environment.Physiol Rev. 2020 Oct 1;100(4):1621-1705. doi: 10.1152/physrev.00041.2019. Epub 2020 Mar 19. Physiol Rev. 2020. PMID: 32191559 Free PMC article. Review.
-
Involvement of ANO1 currents in pacemaking of PDGFRα-positive specialised smooth muscle cells in rat caudal epididymis.Cell Tissue Res. 2024 Jul;397(1):1-12. doi: 10.1007/s00441-024-03890-x. Epub 2024 Apr 8. Cell Tissue Res. 2024. PMID: 38587529
References
-
- Baker SA, Hatton WJ, Han J, et al. Role of TREK-1 potassium channel in bladder overactivity after partial bladder outlet obstruction in mouse. J Urol. 2010;183:793–800. - PubMed
-
- Yoshimura N, Kaiho Y, Miyazato M, et al. Therapeutic receptor targets for lower urinary tract dysfunction. NaunynSchmiedebergs Arch Pharmacol. 2008;377:437–48. - PubMed
-
- Fujiwara M, Andersson K, Persson K. Nitric oxide-induced cGMP accumulation in the mouse bladder is not related to smooth muscle relaxation. Eur J Pharmacol. 2000;401:241–50. - PubMed
-
- McCloskey KD. Interstitial cells of Cajal in the urinary tract. Handb Exp Pharmacol. 2011;202:233–54. - PubMed
-
- Wang XY, Sanders KM, Ward SM. Intimate relationship between interstitial cells of Cajal and enteric nerves in the guinea-pig small intestine. Cell Tissue Res. 1999;295:247–56. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

