Tumor necrosis factor-alpha (TNF-α) is a therapeutic target for impaired cutaneous wound healing
- PMID: 22151742
- PMCID: PMC3287056
- DOI: 10.1111/j.1524-475X.2011.00748.x
Tumor necrosis factor-alpha (TNF-α) is a therapeutic target for impaired cutaneous wound healing
Abstract
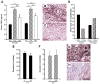
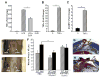
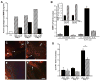
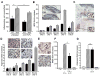
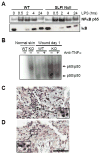
Impaired wound healing states lead to substantial morbidity and cost with treatment resulting in an expenditure of billions of dollars per annum in the U.S. alone. Both chronic wounds and impaired acute wounds are characterized by excessive inflammation, enhanced proteolysis, and reduced matrix deposition. These confounding factors are exacerbated in the elderly, in part, as we report here, related to increased local and systemic tumor necrosis factor-alpha (TNF-α) levels. Moreover, we have used a secretory leukocyte protease inhibitor (SLPI) null mouse model of severely impaired wound healing and excessive inflammation, comparable to age-related delayed human healing, to demonstrate that topical application of anti-TNF-α neutralizing antibodies blunts leukocyte recruitment and NFκB activation, alters the balance between M1 and M2 macrophages, and accelerates wound healing. Following antagonism of TNF-α, matrix synthesis is enhanced, associated with suppression of both inflammatory parameters and NFκB binding activity. Our data suggest that inhibiting TNF-α is a critical event in reversing the severely impaired healing response associated with the absence of SLPI, and may be applicable to prophylaxis and/or treatment of impaired wound healing states in humans.
© 2011 by the Wound Healing Society.
Conflict of interest statement
The authors have no conflicting financial interests.
Figures

References
-
- Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9(8):628–38. - PubMed
-
- Feldmann M, Williams RO, Paleolog E. What have we learnt from targeted anti-TNF therapy? Ann Rheum Dis. 2010;69(Suppl 1):i97–9. - PubMed
-
- Chan JM, Villarreal G, Jin WW, Stepan T, Burstein H, Wahl SM. Intraarticular gene transfer of TNFR:Fc suppresses experimental arthritis with reduced systemic distribution of the gene product. Mol Ther. 2002;6(6):727–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

