Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer
- PMID: 22151962
- PMCID: PMC3326568
- DOI: 10.1186/bcr3072
Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer
Abstract
Introduction: Infiltration of breast tumors by tumor-infiltrating lymphocytes (TIL) has been associated with sensitivity to anthracycline-based chemotherapy. However, it is unclear whether this is true within the estrogen receptor-alpha (ER)-negative subset of breast tumors that frequently manifest high TIL levels.
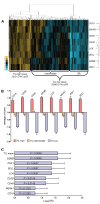
Methods: The association of TIL with short-term and long-term clinical response to anthracycline-based therapy was assessed in two independent ER-negative breast cancer cohorts in which patients were categorized as TIL-high or TIL-low. We defined an eight-gene lymphocyte mRNA expression signature (including CD19, CD3D, CD48, GZMB, LCK, MS4A1, PRF1, and SELL) and used unsupervised hierarchical clustering to examine the association between TIL and short-term response to neoadjuvant chemotherapy in a previously published cohort of ER-negative tumors (n = 113). We also examined the association between TIL and long-term chemotherapeutic efficacy in a second cohort of ER-negative tumors (n = 255) with longer than 6 years of median follow-up by using tissue microarrays and immunohistochemistry (IHC) for detection of CD3, CD8, CD4, CD20, and TIA-1.
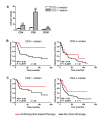
Results: In patients with ER-negative tumors treated with neoadjuvant anthracycline-based chemotherapy, pathologic complete responses (pCRs) were achieved by 23 (74%) of 31 TIL-high patients and 25 (31%) of 80 TIL-low patients (odds ratio (OR), 6.33; 95% confidence interval (CI), 2.49 to 16.08; P < 0.0001). Multivariate logistic regression with standard clinicopathologic features demonstrated that only tumor size (P = 0.037) and TIL status (P = 0.001) were independent predictors of anthracycline response. In the second cohort, adjuvant anthracycline-based therapy was associated with increased disease-free survival (DFS) only in patients with high levels of intraepithelial CD3+ TIL (P = 0.0023). In contrast, outcomes after CMF treatment (cyclophosphamide, methotrexate, and fluorouracil) showed no association with CD3 status. In both cohorts, cytotoxic T-cells were the primary TIL subtype associated with anthracycline sensitivity. Finally, TIL significantly predicted anthracycline sensitivity for both the Her2-positive and triple-negative tumor phenotypes.
Conclusions: ER-negative breast cancers with high levels of TIL have heightened sensitivity to anthracycline-based chemotherapy, as assessed by the immediate response to neoadjuvant therapy and long-term outcome following adjuvant therapy. Investigations of TIL-based predictive tests to identify patients likely to benefit from anthracycline-based treatments are warranted.
Figures

References
-
- Alexe G, Dalgin GS, Scanfeld D, Tamayo P, Mesirov JP, DeLisi C, Harris L, Barnard N, Martel M, Levine AJ, Ganesan S, Bhanot G. High expression of lymphocyte-associated genes in node-negative HER2+ breast cancers correlates with lower recurrence rates. Cancer Res. 2007;67:10669–10676. doi: 10.1158/0008-5472.CAN-07-0539. - DOI - PubMed
-
- Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, Cabaret V, Fermeaux V, Bertheau P, Garnier J, Jeannin JF, Coudert B. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–267. doi: 10.1038/sj.bjc.6602930. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

