Efficacy and onset of action of mometasone furoate/formoterol and fluticasone propionate/salmeterol combination treatment in subjects with persistent asthma
- PMID: 22152089
- PMCID: PMC3298511
- DOI: 10.1186/1710-1492-7-21
Efficacy and onset of action of mometasone furoate/formoterol and fluticasone propionate/salmeterol combination treatment in subjects with persistent asthma
Abstract
Background: Mometasone furoate/formoterol (MF/F) is a novel combination therapy for treatment of persistent asthma. This noninferiority trial compared the effects of MF/F and fluticasone propionate/salmeterol (FP/S) combination therapies on pulmonary function and onset of action in subjects with persistent asthma.
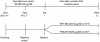
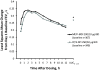
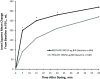
Methods: Following a 2- to 4-week run-in period with MF administered via a metered-dose inhaler (MDI) 200 μg (delivered as 2 inhalations of MF-MDI 100 μg) twice daily (BID), subjects (aged ≥12 y) were randomized to MF/F-MDI 200/10 μg BID (delivered as 2 inhalations of MF/F-MDI 100/5 μg) or FP/S administered via a dry powder inhaler (DPI) 250/50 μg (delivered as 1 inhalation) BID for 12 weeks. The primary assessment was change from baseline to week 12 in area under the curve for forced expiratory volume in 1 second measured serially for 0-12 hours postdose (FEV1 AUC0-12 h). Secondary assessments included onset of action (change from baseline in FEV1 at 5 minutes postdose on day 1) and patient-reported outcomes.
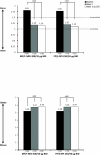
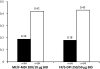
Results: 722 subjects were randomized to MF/F-MDI (n = 371) or FP/S-DPI (n = 351). Mean FEV1 AUC0-12 h change from baseline at week 12 for MF/F-MDI and FP/S-DPI was 3.43 and 3.24 L × h, respectively (95% CI, -0.40 to 0.76). MF/F-MDI was associated with a 200-mL mean increase from baseline in FEV1 at 5 minutes postdose on day 1, which was significantly larger than the 90-mL increase for FP/S-DPI (P < 0.001). The overall incidence of adverse events during the 12-week treatment period that were considered related to study therapy was similar in both groups (MF/F-MDI, 7.8% [n = 29]; FP/S-DPI, 8.3% [n = 29]).
Conclusions: The results of this 12-week study indicated that MF/F improves pulmonary function and asthma control similar to FP/S with a superior onset of action compared with FP/S. Both drugs were safe, improved asthma control, and demonstrated similar results for other secondary study endpoints.
Trial registration: ClinicalTrials.gov: NCT00424008.
Figures
References
-
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. http://www.ginasthma.org/guidelines-gina-report-global-strategy-for-asth... Accessed November 15, 2011.
-
- National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Full Report. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm Accessed December 14, 2010.
-
- Meltzer EO, Kuna P, Nolte H, S Nayak A, Laforce C, on Behalf of the P04073 Study Investigators. Mometasone Furoate/Formoterol Reduces Asthma Deteriorations and Improves Lung Function. Eur Rispirol J. 2011. in press http://erj.ersjournals.com/content/early/2011/08/04/09031936.00020310.long. Accessed Sep 7, 2011. - PubMed
-
- Nathan RA, Nolte H, Pearlman DS. Twenty-six-week efficacy and safety study of mometasone furoate/formoterol 200/10 microg combination treatment in patients with persistent asthma previously receiving medium-dose inhaled corticosteroids. Allergy Asthma Proc. 2010;31(4):269–279. doi: 10.2500/aap.2010.31.3364. - DOI - PubMed
-
- Weinstein SF, Corren J, Murphy K, Nolte H, White M. Twelve-week efficacy and safety study of mometasone furoate/formoterol 200/10 microg and 400/10 microg combination treatments in patients with persistent asthma previously receiving high-dose inhaled corticosteroids. Allergy Asthma Proc. 2010;31(4):280–289. doi: 10.2500/aap.2010.31.3381. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

