Poor quality vital anti-malarials in Africa - an urgent neglected public health priority
- PMID: 22152094
- PMCID: PMC3262771
- DOI: 10.1186/1475-2875-10-352
Poor quality vital anti-malarials in Africa - an urgent neglected public health priority
Abstract
Background: Plasmodium falciparum malaria remains a major public health problem. A vital component of malaria control rests on the availability of good quality artemisinin-derivative based combination therapy (ACT) at the correct dose. However, there are increasing reports of poor quality anti-malarials in Africa.
Methods: Seven collections of artemisinin derivative monotherapies, ACT and halofantrine anti-malarials of suspicious quality were collected in 2002/10 in eleven African countries and in Asia en route to Africa. Packaging, chemical composition (high performance liquid chromatography, direct ionization mass spectrometry, X-ray diffractometry, stable isotope analysis) and botanical investigations were performed.
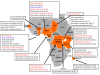
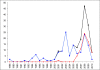
Results: Counterfeit artesunate containing chloroquine, counterfeit dihydroartemisinin (DHA) containing paracetamol (acetaminophen), counterfeit DHA-piperaquine containing sildenafil, counterfeit artemether-lumefantrine containing pyrimethamine, counterfeit halofantrine containing artemisinin, and substandard/counterfeit or degraded artesunate and artesunate+amodiaquine in eight countries are described. Pollen analysis was consistent with manufacture of counterfeits in eastern Asia. These data do not allow estimation of the frequency of poor quality anti-malarials in Africa.
Conclusions: Criminals are producing diverse harmful anti-malarial counterfeits with important public health consequences. The presence of artesunate monotherapy, substandard and/or degraded and counterfeit medicines containing sub-therapeutic amounts of unexpected anti-malarials will engender drug resistance. With the threatening spread of artemisinin resistance to Africa, much greater investment is required to ensure the quality of ACTs and removal of artemisinin monotherapies. The International Health Regulations may need to be invoked to counter these serious public health problems.
Figures
















Similar articles
-
A stratified random survey of the proportion of poor quality oral artesunate sold at medicine outlets in the Lao PDR - implications for therapeutic failure and drug resistance.Malar J. 2009 Jul 28;8:172. doi: 10.1186/1475-2875-8-172. Malar J. 2009. PMID: 19638225 Free PMC article.
-
Got ACTs? Availability, price, market share and provider knowledge of anti-malarial medicines in public and private sector outlets in six malaria-endemic countries.Malar J. 2011 Oct 31;10:326. doi: 10.1186/1475-2875-10-326. Malar J. 2011. PMID: 22039838 Free PMC article.
-
Assessment of the effectiveness of the CD3+ tool to detect counterfeit and substandard anti-malarials.Malar J. 2016 Feb 25;15:119. doi: 10.1186/s12936-016-1180-2. Malar J. 2016. PMID: 26917250 Free PMC article.
-
[Combined antimalarial therapy using artemisinin].Parassitologia. 2004 Jun;46(1-2):85-7. Parassitologia. 2004. PMID: 15305693 Review. Italian.
-
Falsified antimalarials: a minireview.Expert Rev Anti Infect Ther. 2015 Apr;13(4):505-9. doi: 10.1586/14787210.2015.1015990. Epub 2015 Feb 15. Expert Rev Anti Infect Ther. 2015. PMID: 25683870 Review.
Cited by
-
Plasmodium falciparum clearance rates in response to artesunate in Malian children with malaria: effect of acquired immunity.J Infect Dis. 2013 Jun 1;207(11):1655-63. doi: 10.1093/infdis/jit082. Epub 2013 Feb 28. J Infect Dis. 2013. PMID: 23448727 Free PMC article.
-
Subsidizing artemisinin-based combination therapies: a preliminary investigation of the Affordable Medicines Facility - malaria.Res Rep Trop Med. 2012 Jul 17;3:63-68. doi: 10.2147/RRTM.S33690. eCollection 2012. Res Rep Trop Med. 2012. PMID: 30890868 Free PMC article.
-
Identifying market risk for substandard and falsified medicines: an analytic framework based on qualitative research in China, Indonesia, Turkey and Romania.Wellcome Open Res. 2019 Apr 16;4:70. doi: 10.12688/wellcomeopenres.15236.1. eCollection 2019. Wellcome Open Res. 2019. PMID: 31131333 Free PMC article.
-
A tiered analytical approach for investigating poor quality emergency contraceptives.PLoS One. 2014 Apr 18;9(4):e95353. doi: 10.1371/journal.pone.0095353. eCollection 2014. PLoS One. 2014. PMID: 24748219 Free PMC article.
-
Do anti-malarials in Africa meet quality standards? The market penetration of non quality-assured artemisinin combination therapy in eight African countries.Malar J. 2017 May 25;16(1):204. doi: 10.1186/s12936-017-1818-8. Malar J. 2017. PMID: 28539125 Free PMC article.
References
-
- World Health Organization. WHO guidelines for the treatment of malaria. Geneva. 2010. http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf Accessed 12 March 2011.
-
- Arrow KJ, Panosian CB, Gelband H. Saving lives, buying time: Economics of malaria drugs in an age of resistance. Washington (DC): Institute of Medicine of the National Academies; 2004. http://www.nap.edu/books/0309092183/html/ Accessed 12 October 2011. - PubMed
-
- World Health Organization. Country antimalarial drug policies: by region. Geneva. 2009. http://www.who.int/malaria/am_drug_policies_by_region_afro/en/index.html Accessed 12 March 2011.
-
- World Health Organization. Executive Board 124th Session Provisional agenda item 4.11. Counterfeit medical products. EB124. Geneva. 2008. http://www.who.int/gb/ebwha/pdf_files/EB124/B124_14-en.pdf. With corrigendum-see http://apps.who.int/gb/ebwha/pdf_files/EB124/B124_14Corr1-en.pdf. Accessed 12 March 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

