Adaptive Müller cell responses to microglial activation mediate neuroprotection and coordinate inflammation in the retina
- PMID: 22152278
- PMCID: PMC3251543
- DOI: 10.1186/1742-2094-8-173
Adaptive Müller cell responses to microglial activation mediate neuroprotection and coordinate inflammation in the retina
Abstract
Purpose: Microglia and Müller cells are prominent participants in retinal responses to injury and disease that shape eventual tissue adaptation or damage. This investigation examined how microglia and Müller cells interact with each other following initial microglial activation.
Methods: Mouse Müller cells were cultured alone, or co-cultured with activated or unactivated retinal microglia, and their morphological, molecular, and functional responses were evaluated. Müller cell-feedback signaling to microglia was studied using Müller cell-conditioned media. Corroborative in vivo analyses of retinal microglia-Müller cell interactions in the mouse retina were also performed.
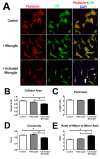
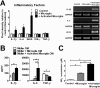
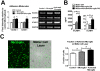
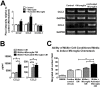
Results: Our results demonstrate that Müller cells exposed to activated microglia, relative to those cultured alone or with unactivated microglia, exhibit marked alterations in cell morphology and gene expression that differed from those seen in chronic gliosis. These Müller cells demonstrated in vitro (1) an upregulation of growth factors such as GDNF and LIF, and provide neuroprotection to photoreceptor cells, (2) increased pro-inflammatory factor production, which in turn increased microglial activation in a positive feedback loop, and (3) upregulated chemokine and adhesion protein expression, which allowed Müller cells to attract and adhere to microglia. In vivo activation of microglia by intravitreal injection of lipopolysaccharide (LPS) also induced increased Müller cell-microglia adhesion, indicating that activated microglia may translocate intraretinally in a radial direction using Müller cell processes as an adhesive scaffold.
Conclusion: Our findings demonstrate that activated microglia are able to influence Müller cells directly, and initiate a program of bidirectional microglia-Müller cell signaling that can mediate adaptive responses within the retina following injury. In the acute aftermath following initial microglia activation, Müller cell responses may serve to augment initial inflammatory responses across retinal lamina and to guide the intraretinal mobilization of migratory microglia using chemotactic cues and adhesive cell contacts. Understanding adaptive microglia-Müller cell interactions in injury responses can help discover therapeutic cellular targets for intervention in retinal disease.
Figures



Similar articles
-
Macroglia-microglia interactions via TSPO signaling regulates microglial activation in the mouse retina.J Neurosci. 2014 Mar 5;34(10):3793-806. doi: 10.1523/JNEUROSCI.3153-13.2014. J Neurosci. 2014. PMID: 24599476 Free PMC article.
-
Electrical stimulation ameliorates light-induced photoreceptor degeneration in vitro via suppressing the proinflammatory effect of microglia and enhancing the neurotrophic potential of Müller cells.Exp Neurol. 2012 Dec;238(2):192-208. doi: 10.1016/j.expneurol.2012.08.029. Epub 2012 Sep 10. Exp Neurol. 2012. PMID: 22974557
-
Microglial-induced Müller cell gliosis is attenuated by progesterone in a mouse model of retinitis pigmentosa.Glia. 2018 Feb;66(2):295-310. doi: 10.1002/glia.23243. Epub 2017 Oct 16. Glia. 2018. PMID: 29034506
-
Microglia-Müller cell interactions in the retina.Adv Exp Med Biol. 2014;801:333-8. doi: 10.1007/978-1-4614-3209-8_42. Adv Exp Med Biol. 2014. PMID: 24664715 Free PMC article. Review.
-
Glia-neuron interactions in the mammalian retina.Prog Retin Eye Res. 2016 Mar;51:1-40. doi: 10.1016/j.preteyeres.2015.06.003. Epub 2015 Jun 23. Prog Retin Eye Res. 2016. PMID: 26113209 Review.
Cited by
-
Jak/Stat signaling regulates the proliferation and neurogenic potential of Müller glia-derived progenitor cells in the avian retina.Sci Rep. 2016 Oct 19;6:35703. doi: 10.1038/srep35703. Sci Rep. 2016. PMID: 27759082 Free PMC article.
-
Chemokine-mediated inflammation in the degenerating retina is coordinated by Müller cells, activated microglia, and retinal pigment epithelium.J Neuroinflammation. 2015 Jan 17;12:8. doi: 10.1186/s12974-014-0224-1. J Neuroinflammation. 2015. PMID: 25595590 Free PMC article.
-
AIBP protects retinal ganglion cells against neuroinflammation and mitochondrial dysfunction in glaucomatous neurodegeneration.Redox Biol. 2020 Oct;37:101703. doi: 10.1016/j.redox.2020.101703. Epub 2020 Aug 27. Redox Biol. 2020. PMID: 32896719 Free PMC article.
-
Proteomic Phenotyping of Stimulated Müller Cells Uncovers Profound Pro-Inflammatory Signaling and Antigen-Presenting Capacity.Front Pharmacol. 2021 Oct 29;12:771571. doi: 10.3389/fphar.2021.771571. eCollection 2021. Front Pharmacol. 2021. PMID: 34776983 Free PMC article.
-
The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration.Int J Mol Sci. 2018 Jan 1;19(1):110. doi: 10.3390/ijms19010110. Int J Mol Sci. 2018. PMID: 29301251 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

