Heterozygosity for a loss-of-function mutation in GALNT2 improves plasma triglyceride clearance in man
- PMID: 22152306
- PMCID: PMC3523677
- DOI: 10.1016/j.cmet.2011.11.005
Heterozygosity for a loss-of-function mutation in GALNT2 improves plasma triglyceride clearance in man
Abstract
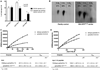
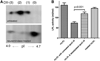
Genome-wide association studies have identified GALNT2 as a candidate gene in lipid metabolism, but it is not known how the encoded enzyme ppGalNAc-T2, which contributes to the initiation of mucin-type O-linked glycosylation, mediates this effect. In two probands with elevated plasma high-density lipoprotein cholesterol and reduced triglycerides, we identified a mutation in GALNT2. It is shown that carriers have improved postprandial triglyceride clearance, which is likely attributable to attenuated glycosylation of apolipoprotein (apo) C-III, as observed in their plasma. This protein inhibits lipoprotein lipase (LPL), which hydrolyses plasma triglycerides. We show that an apoC-III-based peptide is a substrate for ppGalNAc-T2 while its glycosylation by the mutant enzyme is impaired. In addition, neuraminidase treatment of apoC-III which removes the sialic acids from its glycan chain decreases its potential to inhibit LPL. Combined, these data suggest that ppGalNAc-T2 can affect lipid metabolism through apoC-III glycosylation, thereby establishing GALNT2 as a lipid-modifying gene.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Bruneel A, Robert T, Lefeber DJ, Benard G, Loncle E, Djedour A, Durand G, Seta N. Two-dimensional gel electrophoresis of apolipoprotein C-III and other serum glycoproteins for the combined screening of human congenital disorders of O- and N-glycosylation. Proteomics Clin. App. 2007;1:321–324.
-
- Eisenberg S, Bilheimer DW, Levy RI. The metabolism of very low density lipoprotein proteins. II. Studies on the transfer of apoproteins between plasma lipoproteins. Biochim. Biophys. Acta. 1972;280:94–104. - PubMed
-
- Gerken TA, Jamison O, Perrine CL, Collette JC, Moinova H, Ravi L, Markowitz SD, Shen H, Patel H, Tabak LA. Emerging paradigms for the initiation of mucin-type protein O-glycosylation by the polypeptide GalNAc transferase family of glycosyltransferases. J. Biol. Chem. 2011;286:14493–14507. - PMC - PubMed
-
- Hagen FK, Ten Hagen KG, Beres TM, Balys MM, VanWuyckhuyse BC, Tabak LA. cDNA cloning and expression of a novel UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase. J. Biol. Chem. 1997;272:13843–13848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

