Insomnia and objectively measured sleep disturbances predict treatment outcome in depressed patients treated with psychotherapy or psychotherapy-pharmacotherapy combinations
- PMID: 22152403
- PMCID: PMC3310298
- DOI: 10.4088/JCP.11m07184
Insomnia and objectively measured sleep disturbances predict treatment outcome in depressed patients treated with psychotherapy or psychotherapy-pharmacotherapy combinations
Abstract
Objective: Insomnia and objectively measured sleep disturbances predict poor treatment outcomes in patients with major depressive disorder (MDD). However, prior research has utilized individual clinical trials with relatively small sample sizes and has focused on insomnia symptoms or objective measures, but not both. The present study is a secondary analysis that examines the degree to which insomnia, objective sleep disturbances, or their combination predicts depression remission following pharmacotherapy and/or psychotherapy treatment.
Method: Participants were 711 depressed (DSM criteria) patients drawn from 6 clinical trials. Remission status, defined as a score of ≤ 7 on the Hamilton Depression Rating Scale (HDRS) over 2 consecutive months, served as the primary outcome. Insomnia was assessed via the 3 sleep items on the HDRS. Objectively measured short sleep duration (total sleep time ≤ 6 hours) and prolonged sleep latency (> 30 minutes) or wakefulness after sleep onset (> 30 minutes) were derived from in-laboratory polysomnographic sleep studies. Logistic regression predicted the odds of nonremission according to insomnia, each of the objective sleep disturbances, or their combination, after adjusting for age, sex, treatment modality, and baseline depressive symptoms.
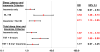
Results: Prolonged sleep latency alone (OR = 3.53; 95% CI, 1.28-9.73) or in combination with insomnia (OR = 2.11; 95% CI, 1.13-3.95) predicted increased risk of nonremission. In addition, insomnia and sleep duration individually and in combination were each associated with a significantly increased risk of nonremission (P values < .05).
Conclusions: Findings suggest that objectively measured prolonged sleep latency and short sleep duration independently or in conjunction with insomnia are risk factors for poor depression treatment outcome.
© Copyright 2012 Physicians Postgraduate Press, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest to disclose with regard to the data presented herein.
Figures


References
-
- Kupfer DJ, Frank E, McEachran AB, et al. Delta sleep ratio: A biological correlate of early recurrence in unipolar affective disorder. Arch Gen Psychiatry. 1990;47:1100–1105. - PubMed
-
- Buysse DJ, Reynolds CF, Hoch CC, et al. Longitudinal effects of nortriptyline on EEG sleep and the likelihood of recurrence in elderly depressed patients. Neuropsychopharmacology. 1996;14:243–252. - PubMed
-
- Kanai T, Takeuchi H, Furukawa TA, et al. Time to recurrence after recovery from major depressive episodes and its predictors. Psychol Med. 2003;33:839–845. - PubMed
-
- Karp JF, Buysse DJ, Houck PR, et al. Relationship of variability in residual symptoms with recurrence of major depressive disorder during mainentance treatment. Am J Psychiatry. 2004;161:1877–1884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH029618/MH/NIMH NIH HHS/United States
- MH-49115/MH/NIMH NIH HHS/United States
- P30 MH071944/MH/NIMH NIH HHS/United States
- R01 MH040023/MH/NIMH NIH HHS/United States
- MH-37869/MH/NIMH NIH HHS/United States
- R01 MH049115/MH/NIMH NIH HHS/United States
- R01 MH037869/MH/NIMH NIH HHS/United States
- R01 MH043832/MH/NIMH NIH HHS/United States
- RR-00056/RR/NCRR NIH HHS/United States
- R37 MH043832/MH/NIMH NIH HHS/United States
- MH71944/MH/NIMH NIH HHS/United States
- P30 MH030915/MH/NIMH NIH HHS/United States
- MH-41884/MH/NIMH NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- R37 MH024652/MH/NIMH NIH HHS/United States
- RR-024153/RR/NCRR NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- M01 RR000056/RR/NCRR NIH HHS/United States
- R01 MH041884/MH/NIMH NIH HHS/United States
- MH-24652/MH/NIMH NIH HHS/United States
- MH-29618/MH/NIMH NIH HHS/United States
- R37 MH029618/MH/NIMH NIH HHS/United States
- R01 MH024652/MH/NIMH NIH HHS/United States
- HL-093220/HL/NHLBI NIH HHS/United States
- MH-43832/MH/NIMH NIH HHS/United States
- MH-30915/MH/NIMH NIH HHS/United States
- K23 HL093220/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

