Beta3-adrenoreceptor stimulation ameliorates myocardial ischemia-reperfusion injury via endothelial nitric oxide synthase and neuronal nitric oxide synthase activation
- PMID: 22152956
- PMCID: PMC3586978
- DOI: 10.1016/j.jacc.2011.09.033
Beta3-adrenoreceptor stimulation ameliorates myocardial ischemia-reperfusion injury via endothelial nitric oxide synthase and neuronal nitric oxide synthase activation
Abstract
Objectives: This paper examined whether nebivolol protects the heart via nitric oxide (NO) synthase and NO-dependent signaling in an in vivo model of acute myocardial infarction.
Background: Beta(3)-adrenergic receptor (AR) activation promotes endothelial nitric oxide synthase (eNOS) activity and NO bioavailability. We hypothesized that specific beta(3)-AR agonists would attenuate myocardial ischemia-reperfusion (MI/R) injury via eNOS activation and increased NO bioavailability.
Methods: Mice were subjected to 45 min of myocardial ischemia in vivo followed by 24 h of reperfusion (R). Nebivolol (500 ng/kg), CL 316243 (1 μg/kg), BRL-37344 (1 μg/kg), or vehicle (VEH) was administered at the time of R. Myocardial area-at-risk (AAR) and infarct size (INF)/AAR was measured at 24 h of R. Cardiac tissue and plasma were collected to evaluate eNOS phosphorylation, neuronal nitric oxide synthase (nNOS), inducible nitric oxide synthase expression, and nitrite and nitrosothiol levels.
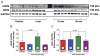
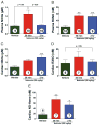
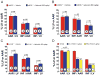
Results: Nebivolol (500 ng/kg) reduced INF/AAR by 37% (p < 0.001 vs. VEH) and serum troponin-I levels from 41 ± 4 ng/ml to 25 ± 4 ng/ml (p < 0.05 vs. VEH). CL 316243 and BRL-37344 reduced INF by 39% and 42%, respectively (p < 0.001 vs. VEH). Nebivolol and CL 316243 increased eNOS phosphorylation at Ser-1177 (p < 0.05 vs. VEH) and increased nitrite and total nitrosylated protein levels. Nebivolol and CL 316243 significantly increased myocardial nNOS expression. Nebivolol failed to reduce INF after MI/R in beta(3)-AR (-/-), eNOS(-/-), and in nNOS(-/-) mice.
Conclusions: Our results indicate that beta(3)-AR agonists protect against MI/R injury. Furthermore, the cardioprotective effects of beta(3)-AR agonists are mediated by rapid eNOS and nNOS activation and increased NO bioavailability.
Copyright © 2011 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures


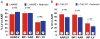
Comment in
-
Beta3-adrenoceptor activation just says NO to myocardial reperfusion injury.J Am Coll Cardiol. 2011 Dec 13;58(25):2692-4. doi: 10.1016/j.jacc.2011.09.034. J Am Coll Cardiol. 2011. PMID: 22152957 No abstract available.
References
-
- Bolli R. Mechanism of myocardial “stunning. Circulation. 1990;82:723–38. - PubMed
-
- Ovize M, Baxter GF, Di Lisa F, et al. Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2010;87:406–23. - PubMed
-
- Heusch G. Postconditioning: old wine in a new bottle? J Am Coll Cardiol. 2004;44:1111–2. - PubMed
-
- Hjalmarson A, Herlitz J, Holmberg S, et al. The Goteborg Metoprolol Trial. Effects on mortality and morbidity in acute myocardial infarction. Circulation. 1983;67:I26–32. - PubMed
-
- Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

