Characterization of a monoclonal anti-capsid antibody that cross-reacts with three major primate lentivirus lineages
- PMID: 22153299
- PMCID: PMC6324184
- DOI: 10.1016/j.virol.2011.11.003
Characterization of a monoclonal anti-capsid antibody that cross-reacts with three major primate lentivirus lineages
Abstract
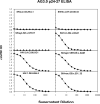
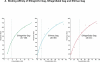
Mouse monoclonal antibodies with varying specificities against the Gag capsid of simian and human immunodeficiency virus (SIV/HIV) were generated by immunizing mice with whole inactivated SIVagmTYO-1. Monoclonal antibody AG3.0 showed the broadest reactivity recognizing the Gag capsid protein (p24-27) and Gag precursors p38, p55, and p150 of HIV-1, HIV-2, SIVmac, and SIVagm. Using overlapping peptides, the AG3.0 epitope was mapped in capsid to a sequence (SPRTLNA) conserved among HIV-1, HIV-2, SIVrcm, SIVsm/mac, and SIVagm related viruses. Because of its broad cross-reactivity, AG3.0 was used to develop an antigen capture assay with a lower detection limit of 100 pg/ml HIV-1 Gag p24. Interestingly, AG3.0 was found to have a faster binding on/off rate for SIVagmVer and SIVmac Gag than for SIVagmSab Gag, possibly due to differences outside the SPRTLNA motif. In addition, the ribonucleic acid (RNA) coding for AG3.0 was sequenced to facilitate the development of humanized monoclonal antibodies.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





References
-
- Aghokeng AF, Bailes E, Loul S, Courgnaud V, Mpoudi-Ngolle E, Sharp PM, Delaporte E, Peeters M. Full-length sequence analysis of SIVmus in wild populations of mustached monkeys (Cercopithecus cephus) from Cameroon provides evidence for two co-circulating SIVmus lineages. Virology. 2007;360(2):407–18. - PMC - PubMed
-
- Allan JS, Whitehead EM, Strout K, Short M, Kanda P, Hart TK, Bugelski PJ. Strong association of simian immunodeficiency virus (SIVagm) envelope glycoprotein heterodimers: possible role in receptor-mediated activation. AIDS Res Hum Retroviruses. 1992;8(12):2011–20. - PubMed
-
- Bailes E, Gao F, Bibollet-Ruche F, Courgnaud V, Peeters M, Marx PA, Hahn BH, Sharp PM. Hybrid origin of SIV in chimpanzees. Science. 2003;300(5626):1713. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

