Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement
- PMID: 22153370
- PMCID: PMC3282061
- DOI: 10.1016/j.neuron.2011.10.028
Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement
Abstract
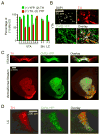
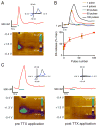
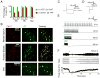
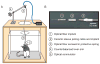
Currently there is no general approach for achieving specific optogenetic control of genetically defined cell types in rats, which provide a powerful experimental system for numerous established neurophysiological and behavioral paradigms. To overcome this challenge we have generated genetically restricted recombinase-driver rat lines suitable for driving gene expression in specific cell types, expressing Cre recombinase under the control of large genomic regulatory regions (200-300 kb). Multiple tyrosine hydroxylase (Th)::Cre and choline acetyltransferase (Chat)::Cre lines were produced that exhibited specific opsin expression in targeted cell types. We additionally developed methods for utilizing optogenetic tools in freely moving rats and leveraged these technologies to clarify the causal relationship between dopamine (DA) neuron firing and positive reinforcement, observing that optical stimulation of DA neurons in the ventral tegmental area (VTA) of Th::Cre rats is sufficient to support vigorous intracranial self-stimulation (ICSS). These studies complement existing targeting approaches by extending the generalizability of optogenetics to traditionally non-genetically-tractable but vital animal models.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc. 2008;3:759–767. - PubMed
-
- Buzsàki G, Bickford RG, Ryan LJ, Young S, Prohaska O, Mandel RJ, Gage FH. Multisite recording of brain field potentials and unit activity in freely moving rats. J Neurosci Methods. 1989;28:209–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

