Parallel regulation of feedforward inhibition and excitation during whisker map plasticity
- PMID: 22153377
- PMCID: PMC3240806
- DOI: 10.1016/j.neuron.2011.09.008
Parallel regulation of feedforward inhibition and excitation during whisker map plasticity
Abstract
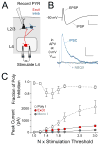
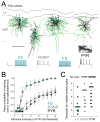
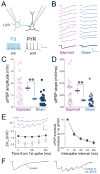
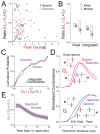
Sensory experience drives robust plasticity of sensory maps in cerebral cortex, but the role of inhibitory circuits in this process is not fully understood. We show that classical deprivation-induced whisker map plasticity in layer 2/3 (L2/3) of rat somatosensory (S1) cortex involves robust weakening of L4-L2/3 feedforward inhibition. This weakening was caused by reduced L4 excitation onto L2/3 fast-spiking (FS) interneurons, which mediate sensitive feedforward inhibition and was partially offset by strengthening of unitary FS to L2/3 pyramidal cell synapses. Weakening of feedforward inhibition paralleled the known weakening of feedforward excitation. As a result, mean excitation-inhibition balance and timing onto L2/3 pyramidal cells were preserved. Thus, reduced feedforward inhibition is a covert compensatory process that can maintain excitatory-inhibitory balance during classical deprivation-induced Hebbian map plasticity.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Allen CB, Celikel T, Feldman DE. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci. 2003;6:291–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

