Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling
- PMID: 22153381
- PMCID: PMC4870892
- DOI: 10.1016/j.neuron.2011.09.028
Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling
Abstract
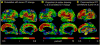
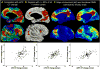
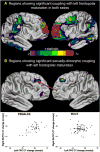
Understanding of human structural brain development has rapidly advanced in recent years, but remains fundamentally "localizational" in nature. Here, we use 376 longitudinally acquired structural brain scans from 108 typically developing adolescents to conduct the first study of coordinated anatomical change within the developing cortex. Correlation in rates of anatomical change was regionally heterogeneous, with fronto-temporal association cortices showing the strongest and most widespread maturational coupling with other cortical areas, and lower-order sensory cortices showing the least. Canonical cortical systems with rich structural and functional interconnectivity showed significantly elevated maturational coupling. Evidence for sexually dimorphic maturational coupling was found within a frontopolar-centered prefrontal system involved in complex decision-making. By providing the first link between cortical connectivity and the coordination of cortical development, we reveal a hitherto unseen property of healthy brain maturation, which may represent a target for neurodevelopmental disease processes, and a substrate for sexually dimorphic behavior in adolescence.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Badre D, Wagner AD. Selection, integration, and conflict monitoring; assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron. 2004;41:473–487. - PubMed
-
- Barton RA, Harvey PH. Mosaic evolution of brain structure in mammals. Nature. 2000;405:1055–1058. - PubMed
-
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. - PubMed
-
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

